Sunitinib therapy for metastatic renal cell carcinoma: recommendations for management of side effects
- PMID: 18542784
- PMCID: PMC2422945
- DOI: 10.5489/cuaj.67
Sunitinib therapy for metastatic renal cell carcinoma: recommendations for management of side effects
Abstract
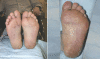
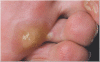
Sunitinib, a new vascular endothelial growth factor receptor inhibitor, has demonstrated high activity in renal cell carcinoma (RCC) and is now widely used for patients with metastatic disease. Although generally well tolerated and associated with a low incidence of common toxicity criteria grade 3 or 4 toxicities, sunitinib exhibits a distinct pattern of novel side effects that require monitoring and management. This article summarizes the most important side effects and proposes recommendations for their monitoring, prevention and treatment, based on the existing literature and on suggestions made by an expert group of Canadian oncologists. Fatigue, diarrhea, anorexia, oral changes, skin toxicity and hypertension seem to be the most clinically relevant toxicities of sunitinib. Fatigue may be partly related to the development of hypothyroidism during sunitinib therapy for which patients should be observed and, if necessary, treated. Hypertension can be treated with standard antihypertensive therapy and rarely requires treatment discontinuation. Neutropenia and thrombocytopenia usually do not require intervention, in particular no episodes of neutropenic fever have been reported to date. A decrease in left ventricular ejection fraction is a rare, but potentially life-threatening side effect. Because of its metabolism by cytochrome P450 3A4 a number of drugs can potentially interact with sunitinib. Clinical response and toxicity should be carefully observed when sunitinib is combined with either a cytochrome P450 3A4 inducer or inhibitor and doses adjusted as necessary. Knowledge about side effects, as well as the proactive assessment and consistent management of sunitinib-related side effects, is critical to ensure optimal benefit from sunitinib treatment.
Figures

References
-
- Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007;356:115-24. - PubMed
-
- Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol 2006;24: 1624. - PubMed
-
- Motzer RJ, Rini BI, Bukowski RM, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA 2006;295:2516-24. - PubMed
-
- Rini BI, Sosman JA, Motzer RJ. Therapy targeted at vascular endothelial growth factor in metastatic renal cell carcinoma: biology, clinical results and future development. BJU Int 2005;96:286-90. - PubMed
-
- Motzer RJ, Bukowski RM. Targeted therapy for metastatic renal cell carcinoma. JClin Oncol 2006;24:5601-8. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
