Regulation of cell cycle entry by PTEN in smooth muscle cell proliferation of human coronary artery bypass conduits
- PMID: 18544045
- PMCID: PMC2782893
- DOI: 10.1111/j.1582-4934.2008.00384.x
Regulation of cell cycle entry by PTEN in smooth muscle cell proliferation of human coronary artery bypass conduits
Abstract
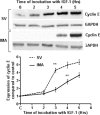
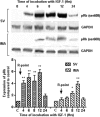
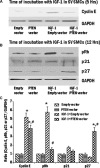
Proliferation of smooth muscle cells (SMCs) is the key event in the pathogenesis of intimal hyperplasia (IH) leading to coronary artery bypass graft (CABG) occlusion. The saphenous vein (SV) conduits are often affected by IH, while the internal mammary artery (IMA) conduits remain remarkably patent. SMC proliferation is mediated by the cell cycle, under the control of cyclin-dependent kinases (cdks), cdk-inhibitors and the retinoblastoma protein (Rb). Early passage of the SMCs through the cell cycle involves crossing the non-reversible G(1) checkpoint, the restriction (R) point. In this study, we investigated the effect of mitogenic insulin-like growth factor (IGF)-1 stimulation on the R-point and its relationship with the phosphorylation of Rb protein and the cdk inhibitors p21 and p27 in SV and IMA SMCs. We observed no change in the R-point following IGF-1 activation in either SV or IMA SMCs. However, Rb-phosphorylation occurred much earlier and was quantitatively greater in SV SMCs than IMA. Overexpression of phosphatase and tensin homologue deleted on chromosome 10 (PTEN) in SV SMCs followed by IGF-1 activation significantly decreased the expression of cyclin E and pRb and induced p27 expression in SV SMCs, while, pRb levels were markedly decreased and p27 levels were significantly increased in IMA SMCs. Silencing the PTEN gene by siRNA transfection of IMA SMCs significantly induced the expression of pRb and inhibited p27 expression, while, the expression levels of cyclin E, pRb, p21 and p27 were unaffected by the silencing of PTEN in SV SMCs. These results demonstrate that the PTEN plays a critical role in regulating cell cycle entry. Therefore, overexpression of PTEN possibly by means of gene therapy could be a viable option in regulating the cell cycle in SV SMCs in the treatment of vein graft disease.
Figures





Similar articles
-
Temporal PTEN inactivation causes proliferation of saphenous vein smooth muscle cells of human CABG conduits.J Cell Mol Med. 2009 Jan;13(1):177-87. doi: 10.1111/j.1582-4934.2008.00311.x. Epub 2008 Mar 19. J Cell Mol Med. 2009. PMID: 18363844 Free PMC article.
-
Insulin-like growth factor-1 induces phosphorylation of PI3K-Akt/PKB to potentiate proliferation of smooth muscle cells in human saphenous vein.Exp Mol Pathol. 2010 Aug;89(1):20-6. doi: 10.1016/j.yexmp.2010.04.002. Epub 2010 May 13. Exp Mol Pathol. 2010. PMID: 20471974 Free PMC article.
-
Smooth muscle cells cultured from human saphenous vein exhibit increased proliferation, invasion, and mitogen-activated protein kinase activation in vitro compared with paired internal mammary artery cells.J Vasc Surg. 2007 May;45(5):1022-8. doi: 10.1016/j.jvs.2007.01.061. J Vasc Surg. 2007. PMID: 17466797
-
Coronary artery bypass graft: why is the saphenous vein prone to intimal hyperplasia?Can J Physiol Pharmacol. 2014 Jul;92(7):531-45. doi: 10.1139/cjpp-2013-0445. Epub 2014 May 16. Can J Physiol Pharmacol. 2014. PMID: 24933515 Review.
-
Cyclins and CDKS in development and cancer: lessons from genetically modified mice.Front Biosci. 2006 Jan 1;11:1164-88. doi: 10.2741/1871. Front Biosci. 2006. PMID: 16146805 Review.
Cited by
-
Integrated systems approach identifies risk regulatory pathways and key regulators in coronary artery disease.J Mol Med (Berl). 2015 Dec;93(12):1381-90. doi: 10.1007/s00109-015-1315-x. Epub 2015 Jul 26. J Mol Med (Berl). 2015. PMID: 26208504
-
Insulin-like growth factor (IGF) binding protein 2 functions coordinately with receptor protein tyrosine phosphatase β and the IGF-I receptor to regulate IGF-I-stimulated signaling.Mol Cell Biol. 2012 Oct;32(20):4116-30. doi: 10.1128/MCB.01011-12. Epub 2012 Aug 6. Mol Cell Biol. 2012. PMID: 22869525 Free PMC article.
-
Apelin inhibits the proliferation and migration of rat PASMCs via the activation of PI3K/Akt/mTOR signal and the inhibition of autophagy under hypoxia.J Cell Mol Med. 2014 Mar;18(3):542-53. doi: 10.1111/jcmm.12208. Epub 2014 Jan 22. J Cell Mol Med. 2014. PMID: 24447518 Free PMC article.
-
Overexpression of the PTEN Gene in Myocardial Tissues of Coronary Bypass Surgery Patients.Arq Bras Cardiol. 2023 Apr 7;120(4):e20220169. doi: 10.36660/abc.20220169. eCollection 2023. Arq Bras Cardiol. 2023. PMID: 37042855 Free PMC article. English, Portuguese.
-
PTEN modulators: a patent review.Expert Opin Ther Pat. 2013 May;23(5):569-80. doi: 10.1517/13543776.2013.768985. Epub 2013 Feb 5. Expert Opin Ther Pat. 2013. PMID: 23379765 Free PMC article. Review.
References
-
- Johnson DG, Walker CL. Cyclins and cell cycle checkpoints. Annu Rev Pharmacol Toxicol. 1999;39:295–312. - PubMed
-
- Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–77. - PubMed
-
- Morgan DO. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol. 1997;13:261–91. - PubMed
-
- Furnari FB, Huang HJ, Cavenee WK. The phosphoinositol phosphatase activity of PTEN mediates a serum-sensitive G1 growth arrest in glioma cells. Cancer Res. 1998;58:5002–8. - PubMed
-
- Li H, Lu S, Fong L. [Study on the status of methylation of Rb gene promoter in human esophageal cancer and effect of NMBzA on Rb gene promoter in monkey esophageal epithelium] Zhonghua Zhong Liu Za Zhi. 1998;20:412–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

