A polymorphism of EGFR extracellular domain is associated with progression free-survival in metastatic colorectal cancer patients receiving cetuximab-based treatment
- PMID: 18544172
- PMCID: PMC2432064
- DOI: 10.1186/1471-2407-8-169
A polymorphism of EGFR extracellular domain is associated with progression free-survival in metastatic colorectal cancer patients receiving cetuximab-based treatment
Abstract
Background: Cetuximab, a monoclonal antibody targeting Epidermal Growth Factor Receptor (EGFR), is currently used in metastatic colorectal cancer (mCRC), but predictive factors for therapeutic response are lacking. Mutational status of KRAS and EGFR, and EGFR copy number are potential determinants of cetuximab activity.
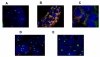
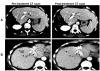
Methods: We analyzed tumor tissues from 32 EGFR-positive mCRC patients receiving cetuximab/irinotecan combination and evaluable for treatment response. EGFR copy number was quantified by fluorescence in situ hybridization (FISH). KRAS exon 1 and EGFR exons coding for extracellular regions were sequenced.
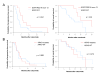
Results: Nine patients experienced an objective response (partial response) and 23 were considered as nonresponders (12 with stable disease and 11 with progressive disease). There was no EGFR amplification found, but high polysomy was noted in 2 patients, both of which were cetuximab responders. No EGFR mutations were found but a variant of exon 13 (R521K) was observed in 12 patients, 11 of which achieved objective response or stable disease. Progression-free and overall survivals were significantly better in patients with this EGFR exon 13 variant. KRAS mutations were found in 14 cases. While there was a trend for an increased KRAS mutation frequency in nonresponder patients (12 mutations out of 23, 52%) as compared to responder patients (2 out of 9, 22%), authentic tumor response or long-term disease stabilization was found in KRAS mutated patients.
Conclusion: This preliminary study suggests that: an increase in EGFR copy number may be associated with cetuximab response but is a rare event in CRC, KRAS mutations are associated with low response rate but do not preclude any cetuximab-based combination efficacy and EGFR exon 13 variant (R521K) may predict for cetuximab benefit.
Figures
Similar articles
-
The importance of KRAS mutations and EGF61A>G polymorphism to the effect of cetuximab and irinotecan in metastatic colorectal cancer.Ann Oncol. 2009 May;20(5):879-84. doi: 10.1093/annonc/mdn712. Epub 2009 Jan 29. Ann Oncol. 2009. PMID: 19179548
-
Epidermal Growth Factor Receptor (EGFR) gene copy number (GCN) correlates with clinical activity of irinotecan-cetuximab in K-RAS wild-type colorectal cancer: a fluorescence in situ (FISH) and chromogenic in situ hybridization (CISH) analysis.BMC Cancer. 2009 Aug 27;9:303. doi: 10.1186/1471-2407-9-303. BMC Cancer. 2009. PMID: 19712476 Free PMC article.
-
Analysis of HER-3, insulin growth factor-1, nuclear factor-kB and epidermal growth factor receptor gene copy number in the prediction of clinical outcome for K-RAS wild-type colorectal cancer patients receiving irinotecan-cetuximab.Ann Oncol. 2012 Jul;23(7):1706-12. doi: 10.1093/annonc/mdr558. Epub 2011 Nov 23. Ann Oncol. 2012. PMID: 22112971
-
Cetuximab in the treatment of patients with colorectal cancer.Expert Opin Biol Ther. 2011 Jul;11(7):937-49. doi: 10.1517/14712598.2011.582464. Epub 2011 May 11. Expert Opin Biol Ther. 2011. PMID: 21557708 Review.
-
Implications for KRAS status and EGFR-targeted therapies in metastatic CRC.Nat Rev Clin Oncol. 2009 Sep;6(9):519-27. doi: 10.1038/nrclinonc.2009.111. Epub 2009 Jul 28. Nat Rev Clin Oncol. 2009. PMID: 19636327 Review.
Cited by
-
Improving Response Rates to EGFR-Targeted Therapies for Head and Neck Squamous Cell Carcinoma: Candidate Predictive Biomarkers and Combination Treatment with Src Inhibitors.J Oncol. 2009;2009:896407. doi: 10.1155/2009/896407. Epub 2009 Jul 14. J Oncol. 2009. PMID: 19636423 Free PMC article.
-
Panitumumab: the evidence for its use in the treatment of metastatic colorectal cancer.Core Evid. 2010 Oct 21;5:61-76. doi: 10.2147/ce.s7035. Core Evid. 2010. PMID: 21042543 Free PMC article.
-
KRAS mutational analysis for colorectal cancer. Application: pharmacogenomic.PLoS Curr. 2010 Sep 2;2:RRN1175. doi: 10.1371/currents.RRN1175. PLoS Curr. 2010. PMID: 20877448 Free PMC article.
-
Epidermal growth factor receptor R521K polymorphism shows favorable outcomes in KRAS wild-type colorectal cancer patients treated with cetuximab-based chemotherapy.Cancer Sci. 2012 Apr;103(4):791-6. doi: 10.1111/j.1349-7006.2012.02225.x. Epub 2012 Feb 22. Cancer Sci. 2012. PMID: 22321154 Free PMC article.
-
EGFR targeted therapy in non-small cell lung cancer: potential role of cetuximab.Biologics. 2009;3:215-24. Epub 2009 Jul 13. Biologics. 2009. PMID: 19707410 Free PMC article.
References
-
- Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. - DOI - PubMed
-
- Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, Canon JL, Van Laethem JL, Maurel J, Richardson G, Wolf M, Amado RG. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–1664. doi: 10.1200/JCO.2006.08.1620. - DOI - PubMed
-
- Lenz HJ, Van Cutsem E, Khambata-Ford S, Mayer RJ, Gold P, Stella P, Mirtsching B, Cohn AL, Pippas AW, Azarnia N, Tsuchihashi Z, Mauro DJ, Rowinsky EK. Multicenter Phase II and Translational Study of Cetuximab in Metastatic Colorectal Carcinoma Refractory to Irinotecan, Oxaliplatin, and Fluoropyrimidines. J Clin Oncol. 2006;24:4914–4921. doi: 10.1200/JCO.2006.06.7595. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

