Hypoxia-inducible factor-2alpha regulates the expression of TRAIL receptor DR5 in renal cancer cells
- PMID: 18544564
- PMCID: PMC2527645
- DOI: 10.1093/carcin/bgn132
Hypoxia-inducible factor-2alpha regulates the expression of TRAIL receptor DR5 in renal cancer cells
Abstract
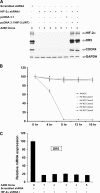
To understand the role of hypoxia-inducible factor (HIF)-2alpha in regulating sensitivity of renal cancer cells to tumor necrosis factor-related apoptosis inducing ligand (TRAIL)-induced apoptosis, we transfected wild-type and mutant von Hippel Lindau (VHL) proteins into TRAIL-sensitive, VHL-negative A498 cells. We find that wild-type VHL, but not the VHL mutants S65W and C162F that do not degrade HIF proteins, cause TRAIL resistance. Knock down of the HIF-2alpha protein by RNA interference (short hairpin RNA) blocked TRAIL-induced apoptosis, decreased the level of TRAIL receptor (DR5) protein and inhibited the transcription of DR5 messenger RNA. By using luciferase constructs containing the upstream region of the DR5 promoter, we demonstrate that HIF-2alpha stimulates the transcription of the DR5 gene by activating the upstream region between -448 and -1188. Because HIF-2alpha is thought to exert its effect on gene transcription by interacting with the Max protein partner of Myc in the Myc/Max dimer, small interfering RNAs to Myc were used to lower the levels of this protein. In multiple renal cancer cell lines decreasing the levels of Myc blocked the ability of HIF-2alpha to stimulate DR5 transcription. PS-341 (VELCADE, bortezomib), a proteasome inhibitor used to treat human cancer, increases the levels of both HIF-2alpha and c-Myc and elevates the level of DR5 in renal cancer, sensitizing renal cancer cells to TRAIL therapy. Similarly, increasing HIF-2alpha in prostate and lung cancer cell lines increased the levels of DR5. Thus, in renal cancer cell lines expressing HIF-2alpha, this protein plays a role in regulating the levels of the TRAIL receptor DR5.
Figures
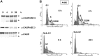




Similar articles
-
Physapubescin selectively induces apoptosis in VHL-null renal cell carcinoma cells through down-regulation of HIF-2α and inhibits tumor growth.Sci Rep. 2016 Sep 1;6:32582. doi: 10.1038/srep32582. Sci Rep. 2016. PMID: 27581364 Free PMC article.
-
Up-regulation of hypoxia-inducible factors HIF-1alpha and HIF-2alpha under normoxic conditions in renal carcinoma cells by von Hippel-Lindau tumor suppressor gene loss of function.Oncogene. 2000 Nov 16;19(48):5435-43. doi: 10.1038/sj.onc.1203938. Oncogene. 2000. PMID: 11114720
-
HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity.Cancer Cell. 2007 Apr;11(4):335-47. doi: 10.1016/j.ccr.2007.02.006. Cancer Cell. 2007. PMID: 17418410 Free PMC article.
-
Hypoxia, Hypoxia-inducible Transcription Factors, and Renal Cancer.Eur Urol. 2016 Apr;69(4):646-657. doi: 10.1016/j.eururo.2015.08.007. Epub 2015 Aug 19. Eur Urol. 2016. PMID: 26298207 Free PMC article. Review.
-
Hypoxia-Inducible Factor in Renal Cell Carcinoma: From Molecular Insights to Targeted Therapies.Genes (Basel). 2024 Dec 24;16(1):6. doi: 10.3390/genes16010006. Genes (Basel). 2024. PMID: 39858553 Free PMC article. Review.
Cited by
-
Tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) in central nervous system inflammation.J Mol Med (Berl). 2009 Aug;87(8):753-63. doi: 10.1007/s00109-009-0484-x. Epub 2009 May 17. J Mol Med (Berl). 2009. PMID: 19449143 Review.
-
Selective expression of tumor necrosis factor-related apoptosis-inducing ligand mediated by microRNA suppresses renal carcinoma growth.Mol Cell Biochem. 2014 Jul;392(1-2):125-34. doi: 10.1007/s11010-014-2025-3. Epub 2014 May 1. Mol Cell Biochem. 2014. PMID: 24788726
-
Death Receptor 5 (TNFRSF10B) Is Upregulated and TRAIL Resistance Is Reversed in Hypoxia and Normoxia in Colorectal Cancer Cell Lines after Treatment with Skyrin, the Active Metabolite of Hypericum spp.Cancers (Basel). 2021 Apr 1;13(7):1646. doi: 10.3390/cancers13071646. Cancers (Basel). 2021. PMID: 33916015 Free PMC article.
-
HIF-2α dictates the susceptibility of pancreatic cancer cells to TRAIL by regulating survivin expression.Oncotarget. 2017 Jun 27;8(26):42887-42900. doi: 10.18632/oncotarget.17157. Oncotarget. 2017. PMID: 28476028 Free PMC article.
-
Physapubescin selectively induces apoptosis in VHL-null renal cell carcinoma cells through down-regulation of HIF-2α and inhibits tumor growth.Sci Rep. 2016 Sep 1;6:32582. doi: 10.1038/srep32582. Sci Rep. 2016. PMID: 27581364 Free PMC article.
References
-
- Jemal A, et al. Cancer statistics, 2003. CA Cancer J. Clin. 2003;53:5–26. - PubMed
-
- Stadler WM. Targeted agents for the treatment of advanced renal cell carcinoma. Cancer. 2005;104:2323–2333. - PubMed
-
- Staehler M, et al. Targeted agents for the treatment of advanced renal cell carcinoma. Curr. Drug Targets. 2005;6:835–846. - PubMed
-
- Griffith TS, et al. Induction and regulation of tumor necrosis factor-related apoptosis-inducing ligand/Apo-2 ligand-mediated apoptosis in renal cell carcinoma. Cancer Res. 2002;62:3093–3099. - PubMed
-
- Pitti RM, et al. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J. Biol. Chem. 1996;271:12687–12690. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

