Disrupting two Arabidopsis thaliana xylosyltransferase genes results in plants deficient in xyloglucan, a major primary cell wall component
- PMID: 18544630
- PMCID: PMC2483363
- DOI: 10.1105/tpc.108.059873
Disrupting two Arabidopsis thaliana xylosyltransferase genes results in plants deficient in xyloglucan, a major primary cell wall component
Abstract
Xyloglucans are the main hemicellulosic polysaccharides found in the primary cell walls of dicots and nongraminaceous monocots, where they are thought to interact with cellulose to form a three-dimensional network that functions as the principal load-bearing structure of the primary cell wall. To determine whether two Arabidopsis thaliana genes that encode xylosyltransferases, XXT1 and XXT2, are involved in xyloglucan biosynthesis in vivo and to determine how the plant cell wall is affected by the lack of expression of XXT1, XXT2, or both, we isolated and characterized xxt1 and xxt2 single and xxt1 xxt2 double T-DNA insertion mutants. Although the xxt1 and xxt2 mutants did not have a gross morphological phenotype, they did have a slight decrease in xyloglucan content and showed slightly altered distribution patterns for xyloglucan epitopes. More interestingly, the xxt1 xxt2 double mutant had aberrant root hairs and lacked detectable xyloglucan. The reduction of xyloglucan in the xxt2 mutant and the lack of detectable xyloglucan in the xxt1 xxt2 double mutant resulted in significant changes in the mechanical properties of these plants. We conclude that XXT1 and XXT2 encode xylosyltransferases that are required for xyloglucan biosynthesis. Moreover, the lack of detectable xyloglucan in the xxt1 xxt2 double mutant challenges conventional models of the plant primary cell wall.
Figures
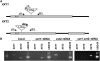
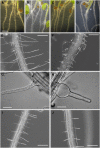

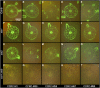

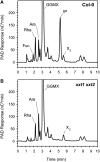

Comment in
-
Role of xyloglucan in primary cell walls.Plant Cell. 2008 Jun;20(6):1421-2. doi: 10.1105/tpc.108.061382. Plant Cell. 2008. PMID: 18559959 Free PMC article. No abstract available.
References
-
- Alonso, J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657. - PubMed
-
- Baskin, T., Betzner, A., Hoggart, R., Cork, A., and Williamson, R. (1992). Root morphology mutants in Arabidopsis thaliana. J. Plant Funct. Biol. 19 427–437.
-
- Bechtold, N., and Bouchez, D. (1994). In planta Agrobacterium-mediated gene transfer by infiltration of adult Arabidopsis thaliana plants by vacuum infiltration. In Gene Transfer to Plants, I. Potrykus and G. Spangenberg, eds (Berlin: Springer-Verlag), pp. 19–23.
-
- Briggs, G.C., Osmont, K.S., Shindo, C., Sibout, R., and Hardtke, C.S. (2006). Unequal genetic redundancies in Arabidopsis - A neglected phenomenon? Trends Plant Sci. 11 492–498. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

