Carcinoembryonic antigen-related cell adhesion molecule 1: a link between insulin and lipid metabolism
- PMID: 18544705
- PMCID: PMC2518480
- DOI: 10.2337/db08-0379
Carcinoembryonic antigen-related cell adhesion molecule 1: a link between insulin and lipid metabolism
Abstract
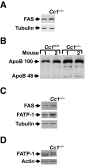
Objective: Liver-specific inactivation of carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) by a dominant-negative transgene (l-SACC1 mice) impaired insulin clearance, caused insulin resistance, and increased hepatic lipogenesis. To discern whether this phenotype reflects a physiological function of CEACAM1 rather than the effect of the dominant-negative transgene, we characterized the metabolic phenotype of mice with null mutation of the Ceacam1 gene (Cc1(-/-)).
Research design and methods: Mice were originally generated on a mixed C57BL/6x129sv genetic background and then backcrossed 12 times onto the C57BL/6 background. More than 70 male mice of each of the Cc1(-/-) and wild-type Cc1(+/+) groups were subjected to metabolic analyses, including insulin tolerance, hyperinsulinemic-euglycemic clamp studies, insulin secretion in response to glucose, and determination of fasting serum insulin, C-peptide, triglyceride, and free fatty acid levels.
Results: Like l-SACC1, Cc1(-/-) mice exhibited impairment of insulin clearance and hyperinsulinemia, which caused insulin resistance beginning at 2 months of age, when the mutation was maintained on a mixed C57BL/6x129sv background, but not until 5-6 months of age on a homogeneous inbred C57BL/6 genetic background. Hyperinsulinemic-euglycemic clamp studies revealed that the inbred Cc1(-/-) mice developed insulin resistance primarily in liver. Despite substantial expression of CEACAM1 in pancreatic beta-cells, insulin secretion in response to glucose in vivo and in isolated islets was normal in Cc1(-/-) mice (inbred and outbred strains).
Conclusions: Intact insulin secretion in response to glucose and impairment of insulin clearance in l-SACC1 and Cc1(-/-) mice suggest that the principal role of CEACAM1 in insulin action is to mediate insulin clearance in liver.
Figures




References
-
- Duckworth WC, Bennett RG, Hamel FG: Insulin degradation: progress and potential. Endocrinol Rev 19 :608 –624,1998 - PubMed
-
- Shillabeer G, Hornford J, Forden JM, Wong NC, Russell JC, Lau DC: Fatty acid synthase and adipsin mRNA levels in obese and lean JCR:LA-cp rats: effect of diet. J Lipid Res 33 :31 –39,1992 - PubMed
-
- Assimacopoulos-Jeannet F, Brichard S, Rencurel F, Cusin I, Jeanrenaud B: In vivo effects of hyperinsulinemia on lipogenic enzymes and glucose transporter expression in rat liver and adipose tissues. Metabolism 44 :228 –233,1995 - PubMed
-
- Rabkin R, Ryan MP, Duckworth WC: The renal metabollism of insulin. Diabetologia 27 :351 –357,1984 - PubMed
-
- Formisano P, Najjar SM, Gross CN, Philippe N, Oriente F, Kern BCL, Accili D, Gorden P: Receptor-mediated internalization of insulin: potential role of pp120/HA4, a substrate of the insulin receptor kinase. J Biol Chem 270 :24073 –24077,1995 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

