Drug metabolism in human brain: high levels of cytochrome P4503A43 in brain and metabolism of anti-anxiety drug alprazolam to its active metabolite
- PMID: 18545703
- PMCID: PMC2408964
- DOI: 10.1371/journal.pone.0002337
Drug metabolism in human brain: high levels of cytochrome P4503A43 in brain and metabolism of anti-anxiety drug alprazolam to its active metabolite
Abstract
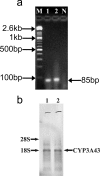
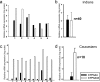
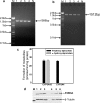
Cytochrome P450 (P450) is a super-family of drug metabolizing enzymes. P450 enzymes have dual function; they can metabolize drugs to pharmacologically inactive metabolites facilitating their excretion or biotransform them to pharmacologically active metabolites which may have longer half-life than the parent drug. The variable pharmacological response to psychoactive drugs typically seen in population groups is often not accountable by considering dissimilarities in hepatic metabolism. Metabolism in brain specific nuclei may play a role in pharmacological modulation of drugs acting on the CNS and help explain some of the diverse response to these drugs seen in patient population. P450 enzymes are also present in brain where drug metabolism can take place and modify therapeutic action of drugs at the site of action. We have earlier demonstrated an intrinsic difference in the biotransformation of alprazolam (ALP) in brain and liver, relatively more alpha-hydroxy alprazolam (alpha-OHALP) is formed in brain as compared to liver. In the present study we show that recombinant CYP3A43 metabolizes ALP to both alpha-OHALP and 4-hydroxy alprazolam (4-OHALP) while CYP3A4 metabolizes ALP predominantly to its inactive metabolite, 4-OHALP. The expression of CYP3A43 mRNA in human brain samples correlates with formation of relatively higher levels of alpha-OH ALP indicating that individuals who express higher levels of CYP3A43 in the brain would generate larger amounts of alpha-OHALP. Further, the expression of CYP3A43 was relatively higher in brain as compared to liver across different ethnic populations. Since CYP3A enzymes play a prominent role in the metabolism of drugs, the higher expression of CYP3A43 would generate metabolite profile of drugs differentially in human brain and thus impact the pharmacodynamics of psychoactive drugs at the site of action.
Conflict of interest statement
Figures



References
-
- Meyer RP, Gehlhaus M, Knoth R, Volk B. Expression and function of cytochrome P450 in brain drug metabolism. Curr Drug Metab. 2007;4:297–306. - PubMed
-
- Haining RL, Nichols HM. Cytochrome P450-catalyzed pathways in human brain: metabolism meets pharmacology or old drugs with new mechanism of action? Pharmacol Ther. 2007;3:537–545. - PubMed
-
- Anandatheerthavarada HK, Shankar SK, Ravindranath V. Rat brain cytochromes P-450: catalytic, immunochemical properties and inducibility of multiple forms. Brain Res. 1990;536:339–343. - PubMed
-
- Pai HV, Upadhya SC, Chinta SJ, Hedge SN, Ravindranath V. Differential metabolism of alprazolam by liver and brain cytochrome (P4503A) to pharmacologically active metabolite. Pharmacogenomics J. 2002;2:243–258. - PubMed
-
- Ravindranath V, Anandatheerthavarada HK, Shankar SK. Xenobiotic metabolism in human brain—presence of cytochrome P-450 and associated mono-oxygenases in human brain regions. Brain Res. 1989;496:331–335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

