Anticancer therapeutics: targeting macromolecules and nanocarriers to hyaluronan or CD44, a hyaluronan receptor
- PMID: 18547053
- PMCID: PMC2772999
- DOI: 10.1021/mp800024g
Anticancer therapeutics: targeting macromolecules and nanocarriers to hyaluronan or CD44, a hyaluronan receptor
Abstract
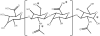
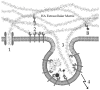
The complex system involved in the synthesis, degradation and binding of the high molecular weight glycosaminoglycan hyaluronic acid (hyaluronan or HA) provides a variety of structures that can be exploited for targeted cancer therapy. In many cancers of epithelial origin there is an upregulation of CD44, a receptor that binds HA. In other cancers, HA in the tumor matrix is overexpressed. Both CD44 on cancer cells and HA in the matrix have been targets for anticancer therapy. Even though CD44 is expressed in normal epithelial cells and HA is part of the matrix of normal tissues, selective targeting to cancer is possible. This is because macromolecular carriers predominantly extravasate into the tumor and not normal tissue; thus CD44-HA targeted carriers administered intravenously localize preferentially into tumors. Anti-CD44 antibodies have been used in patients to deliver radioisotopes or mertansine for treatment of CD44 expressing tumors. In early phase clinical trials, patients with breast or head and neck tumors treated with anti-CD44 conjugates experienced stabilized disease. A dose-limiting toxicity was associated with distribution of the antibody-drug conjugate to the skin, a site in the body with a high level of CD44. HA has been used as a drug carrier and a ligand on liposomes or nanoparticles to target drugs to CD44 overexpressing cells. Drugs can be attached to HA via the carboxylate on the glucuronic acid residue, the hydroxyl on the N-acetylglucosamine or the reducing end which are located on a repeating disaccharide. Drugs delivered in HA-modified liposomes exhibited excellent antitumor activity both in vitro and in murine tumor models. The HA matrix is also a potential target for anticancer therapies. By manipulating the interaction of HA with cell surface receptors, either by degrading it with hyaluronidase or by interfering with CD44-HA interactions using soluble CD44 proteins, tumor progression was blocked. Finally, cytotoxic drugs or prodrug converting enzymes can be attached to the HA matrix to generate a cytotoxic fence around the tumor. This review describes how the complex interplay among cancer biology, the CD44-HA interaction, drug carriers and drug targeting has been used to improve anticancer therapies. As these approaches evolve, they hold forth the prospect of significantly improved targeted anticancer treatments.
Figures

References
-
- Weigel PH, DeAngelis PL. Hyaluronan synthases: a decade-plus of novel glycosyltransferases. J. Biol. Chem. 2007;282:36777–36781. - PubMed
-
- Hua Q, Knudson CB, Knudson W. Internalization of hyaluronan by chondrocytes occurs via receptor-mediated endocytosis. J. Cell. Sci. 1993;106(Pt 1):365–375. - PubMed
-
- Zhou B, Weigel JA, Fauss L, Weigel PH. Identification of the hyaluronan receptor for endocytosis (HARE) J. Biol. Chem. 2000;275:37733–37741. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

