Spatio-temporal modification of collagen scaffolds mediated by triple helical propensity
- PMID: 18547103
- PMCID: PMC3095440
- DOI: 10.1021/bm701378k
Spatio-temporal modification of collagen scaffolds mediated by triple helical propensity
Abstract
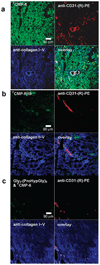
Functionalized collagen that incorporates exogenous compounds may offer new and improved biomaterials applications, especially in drug-delivery, multifunctional implants, and tissue engineering. To that end, we developed a specific and reversible collagen modification technique utilizing associative chain interactions between synthetic collagen mimetic peptide (CMP) [(ProHypGly) chi; Hyp = hydroxyproline] and type I collagen. Here we show temperature-dependent collagen binding and subsequent release of a series of CMPs with varying chain lengths indicating a triple helical propensity driven binding mechanism. The binding took place when melted, single-strand CMPs were allowed to fold while in contact with reconstituted type I collagens. The binding affinity is highly specific to collagen as labeled CMP bound to nanometer scale periodic positions on type I collagen fibers and could be used to selectively image collagens in ex vivo human liver tissue. When heated to physiological temperature, bound CMPs discharged from the collagen at a sustained rate that correlated with CMP's triple helical propensity, suggesting that sustainability is mediated by dynamic collagen-CMP interactions. We also report on the spatially defined modification of collagen film with linear and multi-arm poly(ethylene glycol)-CMP conjugates; at 37 degrees C, these PEG-CMP conjugates exhibited temporary cell repelling activity lasting up to 9 days. These results demonstrate new opportunities for targeting pathologic collagens for diagnostic or therapeutic applications and for fabricating multifunctional collagen coatings and scaffolds that can temporally and spatially control the behavior of cells associated with the collagen matrices.
Figures




Similar articles
-
Template-tethered collagen mimetic peptides for studying heterotrimeric triple-helical interactions.Biopolymers. 2011 Feb;95(2):94-104. doi: 10.1002/bip.21536. Epub 2010 Aug 24. Biopolymers. 2011. PMID: 20740489 Free PMC article.
-
Non-covalent photo-patterning of gelatin matrices using caged collagen mimetic peptides.Macromol Biosci. 2015 Jan;15(1):52-62. doi: 10.1002/mabi.201400436. Epub 2014 Dec 4. Macromol Biosci. 2015. PMID: 25476588 Free PMC article.
-
PEG-based hydrogels with collagen mimetic peptide-mediated and tunable physical cross-links.Biomacromolecules. 2010 Sep 13;11(9):2336-44. doi: 10.1021/bm100465q. Biomacromolecules. 2010. PMID: 20715762 Free PMC article.
-
Targeting and mimicking collagens via triple helical peptide assembly.Curr Opin Chem Biol. 2013 Dec;17(6):968-75. doi: 10.1016/j.cbpa.2013.10.018. Epub 2013 Nov 5. Curr Opin Chem Biol. 2013. PMID: 24210894 Free PMC article. Review.
-
Designed triple-helical peptides as tools for collagen biochemistry and matrix engineering.Philos Trans R Soc Lond B Biol Sci. 2007 Aug 29;362(1484):1281-91. doi: 10.1098/rstb.2007.2115. Philos Trans R Soc Lond B Biol Sci. 2007. PMID: 17581806 Free PMC article. Review.
Cited by
-
HPMA copolymer-collagen hybridizing peptide conjugates targeted to breast tumor extracellular matrix.J Control Release. 2023 Jan;353:278-288. doi: 10.1016/j.jconrel.2022.10.017. Epub 2022 Dec 1. J Control Release. 2023. PMID: 36244509 Free PMC article.
-
Nanoparticle Assembly and Gelatin Binding Mediated by Triple Helical Collagen Mimetic Peptide.ACS Appl Mater Interfaces. 2016 Aug 10;8(31):19907-15. doi: 10.1021/acsami.6b05707. Epub 2016 Jul 27. ACS Appl Mater Interfaces. 2016. PMID: 27403657 Free PMC article.
-
Review collagen-based biomaterials for wound healing.Biopolymers. 2014 Aug;101(8):821-33. doi: 10.1002/bip.22486. Biopolymers. 2014. PMID: 24633807 Free PMC article. Review.
-
Molecular level detection and localization of mechanical damage in collagen enabled by collagen hybridizing peptides.Nat Commun. 2017 Mar 22;8:14913. doi: 10.1038/ncomms14913. Nat Commun. 2017. PMID: 28327610 Free PMC article.
-
Targeting collagen for diagnostic imaging and therapeutic delivery.J Control Release. 2016 Oct 28;240:323-331. doi: 10.1016/j.jconrel.2016.01.007. Epub 2016 Jan 7. J Control Release. 2016. PMID: 26773768 Free PMC article. Review.
References
-
- Lee CH, Singla A, Lee Y. Int. J. Pharm. 2001;221:1–22. - PubMed
-
- Yannas IV. Angew. Chem., Int. Ed. 1990;29:20–35.
-
- Reddi AH. Tissue Eng. 2000;6:351–359. - PubMed
-
- Huynh T, Abraham G, Murray J, Brockbank K, Hagen PO, Sullivan S. Nat. Biotechnol. 1999;17:1083–1086. - PubMed
-
- Huang L, Nagapudi K, Apkarian RP, Chaikof EL. J. Biomater. Sci., Polym. Ed. 2001;12:979–993. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

