Bcl-XL inhibits membrane permeabilization by competing with Bax
- PMID: 18547146
- PMCID: PMC2422857
- DOI: 10.1371/journal.pbio.0060147
Bcl-XL inhibits membrane permeabilization by competing with Bax
Abstract
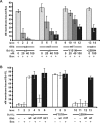
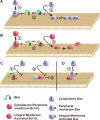
Although Bcl-XL and Bax are structurally similar, activated Bax forms large oligomers that permeabilize the outer mitochondrial membrane, thereby committing cells to apoptosis, whereas Bcl-XL inhibits this process. Two different models of Bcl-XL function have been proposed. In one, Bcl-XL binds to an activator, thereby preventing Bax activation. In the other, Bcl-XL binds directly to activated Bax. It has been difficult to sort out which interaction is important in cells, as all three proteins are present simultaneously. We examined the mechanism of Bax activation by tBid and its inhibition by Bcl-XL using full-length recombinant proteins and measuring permeabilization of liposomes and mitochondria in vitro. Our results demonstrate that Bcl-XL and Bax are functionally similar. Neither protein bound to membranes alone. However, the addition of tBid recruited molar excesses of either protein to membranes, indicating that tBid activates both pro- and antiapoptotic members of the Bcl-2 family. Bcl-XL competes with Bax for the activation of soluble, monomeric Bax through interaction with membranes, tBid, or t-Bid-activated Bax, thereby inhibiting Bax binding to membranes, oligomerization, and membrane permeabilization. Experiments in which individual interactions were abolished by mutagenesis indicate that both Bcl-XL-tBid and Bcl-XL-Bax binding contribute to the antiapoptotic function of Bcl-XL. By out-competing Bax for the interactions leading to membrane permeabilization, Bcl-XL ties up both tBid and Bax in nonproductive interactions and inhibits Bax binding to membranes. We propose that because Bcl-XL does not oligomerize it functions like a dominant-negative Bax in the membrane permeabilization process.
Conflict of interest statement
Figures




References
-
- Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, et al. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. - PubMed
-
- Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. - PubMed
-
- Kim H, Rafiuddin-Shah M, Tu HC, Jeffers JR, Zambetti GP, et al. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat Cell Biol. 2006;8:1348–1358. - PubMed
-
- Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–859. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

