Quantal mEPSCs and residual glutamate: how horizontal cell responses are shaped at the photoreceptor ribbon synapse
- PMID: 18547244
- PMCID: PMC3422860
- DOI: 10.1111/j.1460-9568.2008.06226.x
Quantal mEPSCs and residual glutamate: how horizontal cell responses are shaped at the photoreceptor ribbon synapse
Abstract
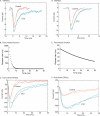
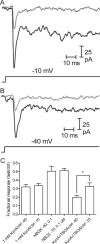
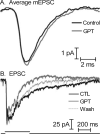
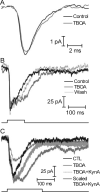
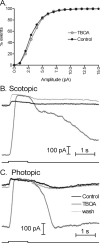
At the photoreceptor ribbon synapse, glutamate released from vesicles at different positions along the ribbon reaches the same postsynaptic receptors. Thus, vesicles may not exert entirely independent effects. We examined whether responses of salamander retinal horizontal cells evoked by light or direct depolarization during paired recordings could be predicted by summation of individual miniature excitatory postsynaptic currents (mEPSCs). For EPSCs evoked by depolarization of rods or cones, linear convolution of mEPSCs with photoreceptor release functions predicted EPSC waveforms and changes caused by inhibiting glutamate receptor desensitization. A low-affinity glutamate antagonist, kynurenic acid (KynA), preferentially reduced later components of rod-driven EPSCs, suggesting lower levels of glutamate are present during the later sustained component of the EPSC. A glutamate-scavenging enzyme, glutamic-pyruvic transaminase, did not inhibit mEPSCs or the initial component of rod-driven EPSCs, but reduced later components of the EPSC. Inhibiting glutamate uptake with a low concentration of DL-threo-beta-benzoyloxyaspartate (TBOA) also did not alter mEPSCs or the initial component of rod-driven EPSCs, but enhanced later components of the EPSC. Low concentrations of TBOA and KynA did not affect the kinetics of fast cone-driven EPSCs. Under both rod- and cone-dominated conditions, light-evoked currents (LECs) were enhanced considerably by TBOA. LECs were more strongly inhibited than EPSCs by KynA, suggesting the presence of lower glutamate levels. Collectively, these results indicate that the initial EPSC component can be largely predicted from a linear sum of individual mEPSCs, but with sustained release, residual amounts of glutamate from multiple vesicles pool together, influencing LECs and later components of EPSCs.
Figures


References
-
- Barbour B, Keller BU, Llano I, Marty A. Prolonged presence of glutamate during excitatory synaptic transmission to cerebellar Purkinje cells. Neuron. 1994;12:1331–1343. - PubMed
-
- Berntson A, Taylor WR. The unitary event amplitude of mouse retinal on-cone bipolar cells. Vis. Neurosci. 2003;20:621–626. - PubMed
-
- Burris C, Klug K, Ngo IT, Sterling P, Schein S. How Muller glial cells in macaque fovea coat and isolate the synaptic terminals of cone photoreceptors. J. Comp. Neurol. 2002;453:100–111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

