A specific cholesterol binding site is established by the 2.8 A structure of the human beta2-adrenergic receptor
- PMID: 18547522
- PMCID: PMC2601552
- DOI: 10.1016/j.str.2008.05.001
A specific cholesterol binding site is established by the 2.8 A structure of the human beta2-adrenergic receptor
Abstract
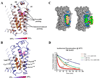
The role of cholesterol in eukaryotic membrane protein function has been attributed primarily to an influence on membrane fluidity and curvature. We present the 2.8 A resolution crystal structure of a thermally stabilized human beta(2)-adrenergic receptor bound to cholesterol and the partial inverse agonist timolol. The receptors pack as monomers in an antiparallel association with two distinct cholesterol molecules bound per receptor, but not in the packing interface, thereby indicating a structurally relevant cholesterol-binding site between helices I, II, III, and IV. Thermal stability analysis using isothermal denaturation confirms that a cholesterol analog significantly enhances the stability of the receptor. A consensus motif is defined that predicts cholesterol binding for 44% of human class A receptors, suggesting that specific sterol binding is important to the structure and stability of other G protein-coupled receptors, and that this site may provide a target for therapeutic discovery.
Figures
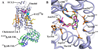

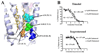

References
-
- Alexandrov AI, Mileni M, Chien EYT, Hanson MA, Stevens RC. Microscale fluorescent thermal stability assay for membrane proteins. Structure. 2008;16:351–359. - PubMed
-
- Baker JG, Hall IP, Hill SJ. Agonist and inverse agonist actions of beta-blockers at the human beta 2-adrenoceptor provide evidence for agonist-directed signaling. Mol. Pharmacol. 2003;64:1357–1369. - PubMed
-
- Ballesteros JA, Weinstein H. Integrated methods for the construction of three dimensional models and computational probing of structure-function relations in G-protein coupled receptors. Methods Neurosci. 1995;25:366–428.
-
- Cherezov V, Peddi A, Muthusubramaniam L, Zheng YF, Caffrey M. A robotic system for crystallizing membrane and soluble proteins in lipidic mesophases. Acta Crystallogr D Biol Crystallogr. 2004;60:1795–1807. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

