Development of a stable, early stage unilateral model of Parkinson's disease in middle-aged rhesus monkeys
- PMID: 18547564
- PMCID: PMC2527750
- DOI: 10.1016/j.expneurol.2008.04.027
Development of a stable, early stage unilateral model of Parkinson's disease in middle-aged rhesus monkeys
Abstract
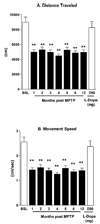
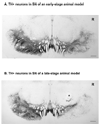
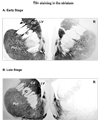
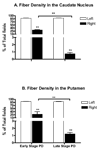
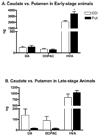
An important issue raised in testing new neuroprotective/restorative treatments for Parkinson's disease (PD) is the optimal stage in the disease process to initiate therapy. Current palliative treatments are effective in the early disease stages raising ethical concerns about substituting an experimental treatment for a proven therapy. Thus, we have endeavored to create a stable 1-methyl-4-phenyl-1,2,3,6 tetrahydropyridine (MPTP) nonhuman primate model of early PD. The new model was created by controlling for dose and route administration of MPTP (unilateral intracarotid infusion), and age of the animals (middleaged, 16-19 years old) in 27 female rhesus monkeys. All animals showed stable parkinsonian features lasting for up to 12-month as per behavioral evaluation. Compared with late-stage PD animals, postmortem analysis demonstrated that more dopaminergic neurons remained in the substantia nigra pars compacta, and more fibers were found in the striatum. In addition, tissue levels of striatal dopamine and its metabolites were also higher. Our results support that a milder but stable PD model can be produced in middle-aged rhesus monkeys.
Figures


Similar articles
-
Overlesioned hemiparkinsonian non human primate model: correlation between clinical, neurochemical and histochemical changes.Front Biosci. 2003 Sep 1;8:a155-66. doi: 10.2741/1104. Front Biosci. 2003. PMID: 12957824
-
Systemic administration of the immunophilin ligand GPI 1046 in MPTP-treated monkeys.Exp Neurol. 2001 Mar;168(1):171-82. doi: 10.1006/exnr.2000.7592. Exp Neurol. 2001. PMID: 11170732
-
Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson's disease.Science. 2000 Oct 27;290(5492):767-73. doi: 10.1126/science.290.5492.767. Science. 2000. PMID: 11052933
-
Time-course of nigrostriatal degeneration in a progressive MPTP-lesioned macaque model of Parkinson's disease.Mol Neurobiol. 2003 Dec;28(3):209-18. doi: 10.1385/MN:28:3:209. Mol Neurobiol. 2003. PMID: 14709785 Review.
-
The intranasal administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a new rodent model to test palliative and neuroprotective agents for Parkinson's disease.Curr Pharm Des. 2011;17(5):489-507. doi: 10.2174/138161211795164095. Curr Pharm Des. 2011. PMID: 21375482 Review.
Cited by
-
Signaling pathways in Parkinson's disease: molecular mechanisms and therapeutic interventions.Signal Transduct Target Ther. 2023 Feb 21;8(1):73. doi: 10.1038/s41392-023-01353-3. Signal Transduct Target Ther. 2023. PMID: 36810524 Free PMC article. Review.
-
Objectively measuring effects of electro-acupuncture in parkinsonian rhesus monkeys.Brain Res. 2018 Jan 1;1678:12-19. doi: 10.1016/j.brainres.2017.10.006. Epub 2017 Oct 7. Brain Res. 2018. PMID: 29017909 Free PMC article.
-
Young and middle-aged rats exhibit isometric forelimb force control deficits in a model of early-stage Parkinson's disease.Behav Brain Res. 2011 Nov 20;225(1):97-103. doi: 10.1016/j.bbr.2011.07.002. Epub 2011 Jul 8. Behav Brain Res. 2011. PMID: 21767573 Free PMC article.
-
Human Clinical-Grade Parthenogenetic ESC-Derived Dopaminergic Neurons Recover Locomotive Defects of Nonhuman Primate Models of Parkinson's Disease.Stem Cell Reports. 2018 Jul 10;11(1):171-182. doi: 10.1016/j.stemcr.2018.05.010. Epub 2018 Jun 14. Stem Cell Reports. 2018. PMID: 29910127 Free PMC article.
-
Improved methods for electroacupuncture and electromyographic recordings in normal and parkinsonian rhesus monkeys.J Neurosci Methods. 2010 Oct 15;192(2):199-206. doi: 10.1016/j.jneumeth.2010.07.016. Epub 2010 Jul 21. J Neurosci Methods. 2010. PMID: 20654649 Free PMC article.
References
-
- Albanese A, Granata R, Gregori B, Piccardi MP, Colosimo C, Tonali P. Chronic administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine to monkeys: behavioural, morphological and biochemical correlates. Neuroscience. 1993;55(3):823–832. - PubMed
-
- Alexander GM, Schwartzman RJ, Brainard L, Gordon SW, Grothusen JR. Changes in brain catecholamines and dopamine uptake sites at different stages of MPTP parkinsonism in monkeys. Brain. Res. 1992;588(2):261–269. - PubMed
-
- Andersen AH, Zhang Z, Zhang M, Gash DM, Avison MJ. Age-associated changes in rhesus CNS composition identified by MRI. Brain. Res. 1999;829(1–2):90–98. - PubMed
-
- Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305(5686):1010–1013. - PubMed
-
- Ballard PA, Tetrud JW, Langston JW. Permanent human parkinsonism due to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): seven cases. Neurology. 1985;35(7):949–956. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

