Synthesis and characterization of a thermally-responsive tumor necrosis factor antagonist
- PMID: 18547669
- PMCID: PMC2637771
- DOI: 10.1016/j.jconrel.2008.04.021
Synthesis and characterization of a thermally-responsive tumor necrosis factor antagonist
Abstract
Numerous antagonists of tumor necrosis factor alpha (TNFalpha) have been developed to attenuate inflammation and accompanying pain in many disease processes. Soluble TNF receptor type II (sTNFRII) is one such antagonist that sequesters TNFalpha away from target receptors and attenuates its activity. Systemic delivery of soluble TNF receptors or other antagonists may have deleterious side effects associated with immune suppression, so that strategies for locally targeted drug delivery are of interest. Elastin-like polypeptides (ELPs) are biopolymers capable of in situ drug depot formation through thermally-driven supramolecular complexes at physiological temperatures. A recombinant fusion protein between ELP and sTNFRII was designed and evaluated for retention of bivalent functionality. Thermal sensitivity was observed by formation of supramolecular submicron-sized particles at 32 degrees C, with gradual resolubilization from the depot observed at physiological temperatures. In vitro refolding of the sTNFRII domain was required and the purified product exhibited an equilibrium dissociation constant for interacting with TNFalpha that was seven-fold higher than free sTNFRII. Furthermore, anti-TNF activity was observed in inhibiting TNFalpha-mediated cytotoxicity in the murine L929 fibrosarcoma assay. Potential advantages of this ELP-sTNFRII fusion protein as an anti-TNFa drug depot include facility of injection, in situ depot formation, low endotoxin content, and functionality against TNFalpha.
Figures





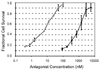
 ) with an IC50 of 12 ± 4 nM. Coefficients of determination, r2, for fitting to a dose-response curve (Equation 2) ranged from 0.94 to 0.99.
) with an IC50 of 12 ± 4 nM. Coefficients of determination, r2, for fitting to a dose-response curve (Equation 2) ranged from 0.94 to 0.99.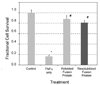
References
-
- Dayer JM. The saga of the discovery of IL-1 and TNF and their specific inhibitors in the pathogenesis and treatment of rheumatoid arthritis. Joint Bone Spine. 2002;69(2):123–132. - PubMed
-
- Feldmann M, Maini RN. Lasker Clinical Medical Research Award. TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases. Nature medicine. 2003;9(10):1245–1250. - PubMed
-
- Maini RN, Elliott M, Brennan FM, Williams RO, Feldmann M. TNF blockade in rheumatoid arthritis: implications for therapy and pathogenesis. Apmis. 1997;105(4):257–263. - PubMed
-
- Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflammatory bowel diseases. 2006;12 Suppl 1:S3–S9. - PubMed
-
- Neurath MF, Fuss I, Pasparakis M, Alexopoulou L, Haralambous S, Meyer zum Buschenfelde KH, Strober W, Kollias G. Predominant pathogenic role of tumor necrosis factor in experimental colitis in mice. European journal of immunology. 1997;27(7):1743–1750. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

