Deregulation of sphingolipid metabolism in Alzheimer's disease
- PMID: 18547682
- PMCID: PMC2829762
- DOI: 10.1016/j.neurobiolaging.2008.05.010
Deregulation of sphingolipid metabolism in Alzheimer's disease
Abstract
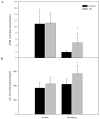
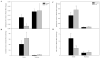
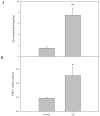
Abnormal sphingolipid metabolism has been previously reported in Alzheimer's disease (AD). To extend these findings, several sphingolipids and sphingolipid hydrolases were analyzed in brain samples from AD patients and age-matched normal individuals. We found a pattern of elevated acid sphingomyelinase (ASM) and acid ceramidase (AC) expression in AD, leading to a reduction in sphingomyelin and elevation of ceramide. More sphingosine also was found in the AD brains, although sphingosine-1-phosphate (S1P) levels were reduced. Notably, significant correlations were observed between the brain ASM and S1P levels and the levels of amyloid beta (Abeta) peptide and hyperphosphorylated tau protein. Based on these findings, neuronal cell cultures were treated with Abeta oligomers, which were found to activate ASM, increase ceramide, and induce apoptosis. Pre-treatment of the neurons with purified, recombinant AC prevented the cells from undergoing Abeta-induced apoptosis. We propose that ASM activation is an important pathological event leading to AD, perhaps due to Abeta deposition. The downstream consequences of ASM activation are elevated ceramide, activation of ceramidases, and production of sphingosine. The reduced levels of S1P in the AD brain, together with elevated ceramide, likely contribute to the disease pathogenesis.
Copyright 2008 Elsevier Inc. All rights reserved.
Figures



References
-
- Alessenko AV, Bugrova AE, Dudnik LB. Connection of lipid peroxide oxidation with the sphingomyelin pathway in the development of Alzheimer's disease. Biochem. Soc. Trans. 2004;32:144–146. - PubMed
-
- Ayasolla K, Khan M, Singh AK, Singh I. Inflammatory mediator and beta-amyloid (25-35)-induced ceramide generation and iNOS expression are inhibited by vitamin E. Free Radic. Biol. Med. 2004;37:325–338. - PubMed
-
- Bollinger CR, Teichgraber V, Gulbins E. Ceramide-enriched membrane domains. Biochim. Biophys. Acta. 2005;1746:284–294. - PubMed
-
- Buchet R, Pikula S. Alzheimer's disease: its origin at the membrane, evidence and questions. Acta Biochim. Pol. 2000;47:725–733. - PubMed
-
- Chen H, Tung YC, Li B, Iqbal K, Grundke-Iqbal I. Trophic factors counteract elevated FGF-2-induced inhibition of adult neurogenesis. Neurobiol. Aging. 2007;28:1148–1162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

