Amine-directed hydroboration: scope and limitations
- PMID: 18549217
- PMCID: PMC2646876
- DOI: 10.1021/ja0774663
Amine-directed hydroboration: scope and limitations
Abstract
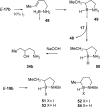
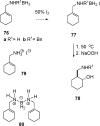
Iodine activation induces intramolecular hydroboration of homoallylic and bis-homoallylic amine boranes with good to excellent control of regiochemistry compared to control experiments using excess THF*BH 3. Deuterium labeling and other evidence confirm that the iodine-induced hydroboration reaction of homoallylic amine boranes occurs via an intramolecular mechanism equivalent to the classical 4-center process and without competing retro-hydroboration. Longer carbon chain tethers result in lower regioselectivity, whereas the shorter tether in allylic amines results in a switch to dominant intermolecular hydroboration. Regioselectivity in THF*BH 3 control experiments is higher for the allylic amine boranes compared to the iodine activation experiments, whereas the reverse is true for homoallylic amine borane activation.
Figures
References
-
- Dewar M. J. S.; Gleicher G. J.; Robinson B. P. J. Am. Chem. Soc. 1964, 86, 5698.
- Dewar M. J. S.; Rona P. J. Am. Chem. Soc. 1967, 89, 6294.
- Polívka Z.; Ferles M. Collect. Czech. Chem. Commun. 1969, 34, 3009.
- Polívka Z.; Kubelka V.; Holubová N.; Ferles M. Collect. Czech. Chem. Commun. 1970, 35, 1131.
- Wille H.; Goubeau J. Chem. Ber. 1972, 105, 2156.
- Baboulene M.; Torregrosa J.-L.; Speziale V.; Lattes A. Bull. Chim. Soc. Fr. 1980, II-565.
- Midland M, M.; Kazubski A. J. Org. Chem. 1992, 57, 2953.
-
- Brown H. C.; Chandrasekharan J. J. Am. Chem. Soc. 1984, 106, 1863.
-
- Brown H. C.; Bartholomay H. Jr; Taylor M. D. J. Am. Chem. Soc. 1944, 66, 435.
- Haaland A. Angew. Chem, Int. Ed. Engl. 1989, 28, 992.
- Brown H. C.; Kanth J. V. B.; Dalvi P. V.; Zaidlewicz M. J. Org. Chem. 2000, 65, 4655. - PubMed
-
- Schmidbaur H.; Sigl M.; Schier A. J. Organomet. Chem. 1997, 529, 323.
- Gaumont A.-C.-C.; Bourumeau K.; Denis J.-M.; Guenot P J. Organomet. Chem. 1994, 484, 9.
-
- Hoveyda A. H.; Evans D. A.; Fu G. C. Chem. Rev. 1993, 93, 1307.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous