A second conserved GAF domain cysteine is required for the blue/green photoreversibility of cyanobacteriochrome Tlr0924 from Thermosynechococcus elongatus
- PMID: 18549244
- PMCID: PMC2574597
- DOI: 10.1021/bi800088t
A second conserved GAF domain cysteine is required for the blue/green photoreversibility of cyanobacteriochrome Tlr0924 from Thermosynechococcus elongatus
Abstract
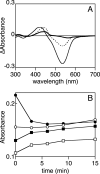
Phytochromes are widely occurring red/far-red photoreceptors that utilize a linear tetrapyrrole (bilin) chromophore covalently bound within a knotted PAS-GAF domain pair. Cyanobacteria also contain more distant relatives of phytochromes that lack this knot, such as the phytochrome-related cyanobacteriochromes implicated to function as blue/green switchable photoreceptors. In this study, we characterize the cyanobacteriochrome Tlr0924 from the thermophilic cyanobacterium Thermosynechococcus elongatus. Full-length Tlr0924 exhibits blue/green photoconversion across a broad range of temperatures, including physiologically relevant temperatures for this organism. Spectroscopic characterization of Tlr0924 demonstrates that its green-absorbing state is in equilibrium with a labile, spectrally distinct blue-absorbing species. The photochemically generated blue-absorbing state is in equilibrium with another species absorbing at longer wavelengths, giving a total of 4 states. Cys499 is essential for this behavior, because mutagenesis of this residue results in red-absorbing mutant biliproteins. Characterization of the C 499D mutant protein by absorbance and CD spectroscopy supports the conclusion that its bilin chromophore adopts a similar conformation to the red-light-absorbing P r form of phytochrome. We propose a model photocycle in which Z/ E photoisomerization of the 15/16 bond modulates formation of a reversible thioether linkage between Cys499 and C10 of the chromophore, providing the basis for the blue/green switching of cyanobacteriochromes.
Figures







References
-
- Briggs WR, Spudich JA, editors. Handbook of Photosensory Receptors. Wiley VCH; Weinheim: 2005.
-
- Schäfer E, Nagy F, editors. Photomorphogenesis in Plants and Bacteria: Function and Signal Transduction Mechanisms. 3rd Edition Springer, Dordrecht; The Netherlands: 2005.
-
- Montgomery BL, Lagarias JC. Phytochrome ancestry. Sensors of bilins and light. Tr. Plant Sci. 2002;7:357–366. - PubMed
-
- Gärtner W, Braslavsky SE. The phytochromes: Spectroscopy and function. In: Batschauer A, editor. Photoreceptors and Light Signalling. Royal Society of Chemistry; Cambridge, UK: 2003. pp. 136–180.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

