Molecular basis of kainate receptor modulation by sodium
- PMID: 18549784
- PMCID: PMC2575105
- DOI: 10.1016/j.neuron.2008.04.001
Molecular basis of kainate receptor modulation by sodium
Abstract
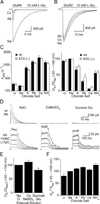
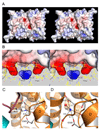
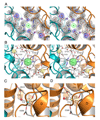
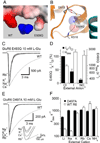
Membrane proteins function in a polarized ionic environment with sodium-rich extracellular and potassium-rich intracellular solutions. Glutamate receptors that mediate excitatory synaptic transmission in the brain show unusual sensitivity to external ions, resulting in an apparent requirement for sodium in order for glutamate to activate kainate receptors. Here, we solve the structure of the Na(+)-binding sites and determine the mechanism by which allosteric anions and cations regulate ligand-binding dimer stability, and hence the rate of desensitization and receptor availability for gating by glutamate. We establish a stoichiometry for binding of 2 Na(+) to 1 Cl(-) and show that allosteric anions and cations bind at physically discrete sites with strong electric fields, that the binding sites are not saturated in CSF, and that the requirement of kainate receptors for Na(+) occurs simply because other cations bind with lower affinity and have lower efficacy compared to Na(+).
Figures


References
-
- Alvarez O, Gonzalez C, Latorre R. Counting channels: a tutorial guide on ion channel fluctuation analysis. Adv Physiol Educ. 2002;26:327–341. - PubMed
-
- Armstrong N, Gouaux E. Mechanisms for activation and antagonism of an AMPA-sensitive glutamate receptor: Crystal structures of the GluR2 ligand binding core. Neuron. 2000;28:165–181. - PubMed
-
- Armstrong N, Jasti J, Beich-Frandsen M, Gouaux E. Measurement of conformational changes accompanying desensitization in an ionotropic glutamate receptor. Cell. 2006;127:85–97. - PubMed
-
- Berendsen HJC, Postma JPM, van Gunsteren WF, DiNola A, Haak JR. Molecular dynamics with coupling to an external bath. The Journal of Chemical Physics. 1984;81:3684–3690.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

