Carbon monoxide, reactive oxygen signaling, and oxidative stress
- PMID: 18549826
- PMCID: PMC2570053
- DOI: 10.1016/j.freeradbiomed.2008.05.013
Carbon monoxide, reactive oxygen signaling, and oxidative stress
Abstract
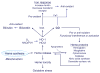
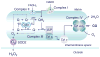
The ubiquitous gas, carbon monoxide (CO), is of substantial biological importance, but apart from its affinity for reduced transition metals, particularly heme-iron, it is surprisingly nonreactive-as is the ferrous-carbonyl-in living systems. CO does form strong complexes with heme proteins for which molecular O2 is the preferred ligand and to which are attributed diverse physiological, adaptive, and toxic effects. Lately, it has become apparent that both exogenous and endogenous CO produced by heme oxygenase engender a prooxidant milieu in aerobic mammalian cells which initiates signaling related to reactive oxygen species (ROS) generation. ROS signaling contingent on CO can be segregated by CO concentration-time effects on cellular function, by the location of heme proteins, e.g., mitochondrial or nonmitochondrial sites, or by specific oxidation-reduction (redox) reactions. The fundamental responses to CO involve overt physiological regulatory events, such as activation of redox-sensitive transcription factors or stress-activated kinases, which institute compensatory expression of antioxidant enzymes and other adaptations to oxidative stress. In contrast, responses originating from highly elevated or protracted CO exposures tend to be nonspecific, produce untoward biological oxidations, and interfere with homeostasis. This brief overview provides a conceptual framework for understanding CO biology in terms of this physiological-pathological hierarchy.
Figures



References
-
- Sjostrand T. Early studies of CO production. Ann N Y Acad Sci. 1970;174:5–10. - PubMed
-
- Maines MD. The heme oxygenase system: update 2005. Antioxid Redox Signal. 2005;7:1761–6. - PubMed
-
- Caughey WS. Carbon monoxide bonding in hemeproteins. Ann N Y Acad Sci. 1970;174:148–53. - PubMed
-
- Piantadosi CA. Biological chemistry of carbon monoxide. Antioxid Redox Signal. 2002;4:259–70. - PubMed
-
- Boczkowski J, Poderoso JJ, Motterlini R. CO-metal interaction: Vital signaling from a lethal gas. Trends Biochem Sci. 2006;31:614–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

