A transgenic sensor strain for monitoring the RNAi pathway in the yellow fever mosquito, Aedes aegypti
- PMID: 18549956
- PMCID: PMC2518454
- DOI: 10.1016/j.ibmb.2008.04.002
A transgenic sensor strain for monitoring the RNAi pathway in the yellow fever mosquito, Aedes aegypti
Abstract
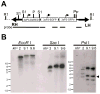
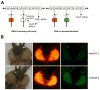
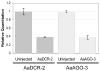
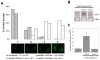
The RNA interference pathway functions as an antiviral defense in invertebrates. In order to generate a phenotypic marker which "senses" the status of the RNAi pathway in Aedes aegypti, transgenic strains were developed to express EGFP and DsRED marker genes in the eye, as well as double-stranded RNA homologous to a portion of the EGFP gene. Transgenic "sensor" mosquitoes exhibited robust eye-specific DsRED expression with little EGFP, indicating RNAi-based silencing. Cloning and high-throughput sequencing of small RNAs confirmed that the inverted-repeat transgene was successfully processed into short-interfering RNAs by the mosquito RNAi pathway. When the A. aegypti homologues of the genes DCR-2 or AGO-2 were knocked down, a clear increase in EGFP fluorescence was observed in the mosquito eyes. Knockdown of DCR-2 was also associated with an increase in EGFP mRNA levels, as determined by Northern blot and real-time PCR. Knockdown of AGO-3, a gene involved in the germline-specific piRNA pathway, did not restore EGFP expression at either the mRNA or protein level. This transgenic sensor strain can now be used to identify other components of the mosquito RNAi pathway and has the potential to be used in the identification of arboviral suppressors of RNAi.
Figures
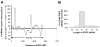
References
-
- Adelman ZN, Jasinskiene N, James AA. Development and applications of transgenesis in the yellow fever mosquito, Aedes aegypti. Mol Biochem Parasitol. 2002a;121:1–10. - PubMed
-
- Adelman ZN, Jasinskiene N, Vally KJ, Peek C, Travanty EA, Olson KE, Brown SE, Stephens JL, Knudson DL, Coates CJ, James AA. Formation and loss of large, unstable tandem arrays of the piggyBac transposable element in the yellow fever mosquito, Aedes aegypti. Transgenic Res. 2004;13:411–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

