The pilocarpine model of temporal lobe epilepsy
- PMID: 18550176
- PMCID: PMC2518220
- DOI: 10.1016/j.jneumeth.2008.04.019
The pilocarpine model of temporal lobe epilepsy
Abstract
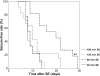
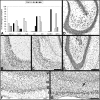
Understanding the pathophysiogenesis of temporal lobe epilepsy (TLE) largely rests on the use of models of status epilepticus (SE), as in the case of the pilocarpine model. The main features of TLE are: (i) epileptic foci in the limbic system; (ii) an "initial precipitating injury"; (iii) the so-called "latent period"; and (iv) the presence of hippocampal sclerosis leading to reorganization of neuronal networks. Many of these characteristics can be reproduced in rodents by systemic injection of pilocarpine; in this animal model, SE is followed by a latent period and later by the appearance of spontaneous recurrent seizures (SRSs). These processes are, however, influenced by experimental conditions such as rodent species, strain, gender, age, doses and routes of pilocarpine administration, as well as combinations with other drugs administered before and/or after SE. In the attempt to limit these sources of variability, we evaluated the methodological procedures used by several investigators in the pilocarpine model; in particular, we have focused on the behavioural, electrophysiological and histopathological findings obtained with different protocols. We addressed the various experimental approaches published to date, by comparing mortality rates, onset of SRSs, neuronal damage, and network reorganization. Based on the evidence reviewed here, we propose that the pilocarpine model can be a valuable tool to investigate the mechanisms involved in TLE, and even more so when standardized to reduce mortality at the time of pilocarpine injection, differences in latent period duration, variability in the lesion extent, and SRS frequency.
Figures
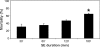

References
-
- Amado D., Cavalheiro E.A. Hormonal and gestational parameters in female rats submitted to the pilocarpine model of epilepsy. Epilepsy Res. 1998;32:266–274. - PubMed
-
- André V., Dubé C., François J., Leroy C., Rigoulot M.A., Roch C. Pathogenesis and pharmacology of epilepsy in the lithium–pilocarpine model. Epilepsia. 2007;48(Suppl 5):41–47. - PubMed
-
- Arida R.M., Scorza F.A., Peres C.A., Cavalheiro E.A. The course of untreated seizures in the pilocarpine model of epilepsy. Epilepsy Res. 1999;34:99–107. - PubMed
-
- Avoli M., D’Antuono M., Louvel J., Kohling R., Biagini G., Pumain R. Network and pharmacological mechanisms leading to epileptiform synchronization in the limbic system in vitro. Prog Neurobiol. 2002;68:167–207. - PubMed
-
- Bartolomei F., Khalil M., Wendling F., Sontheimer A., Regis J., Ranjeva J.P. Entorhinal cortex involvement in human mesial temporal lobe epilepsy: an electrophysiologic and volumetric study. Epilepsia. 2005;46:677–687. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

