TRPA1 mediates the noxious effects of natural sesquiterpene deterrents
- PMID: 18550530
- PMCID: PMC2527119
- DOI: 10.1074/jbc.M710280200
TRPA1 mediates the noxious effects of natural sesquiterpene deterrents
Abstract
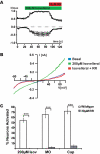
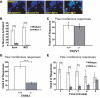
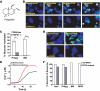
Plants, fungi, and animals generate a diverse array of deterrent natural products that induce avoidance behavior in biological adversaries. The largest known chemical family of deterrents are terpenes characterized by reactive alpha,beta-unsaturated dialdehyde moieties, including the drimane sesquiterpenes and other terpene species. Deterrent sesquiterpenes are potent activators of mammalian peripheral chemosensory neurons, causing pain and neurogenic inflammation. Despite their wide-spread synthesis and medicinal use as desensitizing analgesics, their molecular targets remain unknown. Here we show that isovelleral, a noxious fungal sesquiterpene, excites sensory neurons through activation of TPRA1, an ion channel involved in inflammatory pain signaling. TRPA1 is also activated by polygodial, a drimane sesquiterpene synthesized by plants and animals. TRPA1-deficient mice show greatly reduced nocifensive behavior in response to isovelleral, indicating that TRPA1 is the major receptor for deterrent sesquiterpenes in vivo. Isovelleral and polygodial represent the first fungal and animal small molecule agonists of nociceptive transient receptor potential channels.
Figures


References
-
- Gershenzon, J., and Dudareva, N. (2007) Nat. Chem. Biol. 3 408-414 - PubMed
-
- Langenheim, J. H. (1994) J. Chem. Ecol. 20 1223-1280 - PubMed
-
- List, P. H., and Hackenberg, H. (1969) Arch. Pharm. 302 125-143 - PubMed
-
- Magnusson, G., Thoren, S., and Wickberg, B. (1972) Tetrahedron Lett. 13 1105-1108
-
- Camazine, S. M., Resch, J. F., Eisner, T., and Meinwald, J. (1983) J. Chem. Ecol. 9 1439-1447 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

