Manganese-enhanced MRI: an exceptional tool in translational neuroimaging
- PMID: 18550591
- PMCID: PMC2632451
- DOI: 10.1093/schbul/sbn056
Manganese-enhanced MRI: an exceptional tool in translational neuroimaging
Abstract
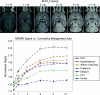
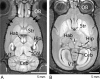
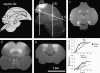
The metal manganese is a potent magnetic resonance imaging (MRI) contrast agent that is essential in cell biology. Manganese-enhanced magnetic resonance imaging (MEMRI) is providing unique information in an ever-growing number of applications aimed at understanding the anatomy, the integration, and the function of neural circuits both in normal brain physiology as well as in translational models of brain disease. A major drawback to the use of manganese as a contrast agent, however, is its cellular toxicity. Therefore, paramount to the successful application of MEMRI is the ability to deliver Mn2+ to the site of interest using as low a dose as possible while preserving detectability by MRI. In the present work, the different approaches to MEMRI in translational neuroimaging are reviewed and challenges for future identified from a practical standpoint.
Figures
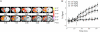
References
-
- Li Y, Huang TT, Carlson EJ, et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995;11(4):376–381. - PubMed
-
- Gunter TE, Gavin CE, Aschner M, Gunter KK. Speciation of manganese in cells and mitochondria: a search for the proximal cause of manganese neurotoxicity. Neurotoxicology. 2006;27(5):765–776. - PubMed
-
- Zwingmann C, Leibfritz D, Hazell AS. Brain energy metabolism in a sub-acute rat model of manganese neurotoxicity: an ex vivo nuclear magnetic resonance study using [1-13C]glucose. Neurotoxicology. 2004;25(4):573–587. - PubMed
-
- Wedler FC, Denman RB. Glutamine synthetase: the major Mn(II) enzyme in mammalian brain. Curr Top Cell Regul. 1984;24:153–169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

