Synapsin- and actin-dependent frequency enhancement in mouse hippocampal mossy fiber synapses
- PMID: 18550596
- PMCID: PMC2638812
- DOI: 10.1093/cercor/bhn101
Synapsin- and actin-dependent frequency enhancement in mouse hippocampal mossy fiber synapses
Abstract
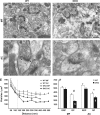
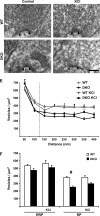
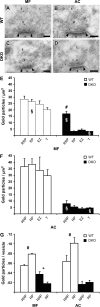
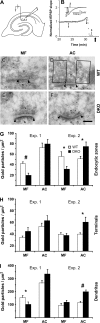
The synapsin proteins have different roles in excitatory and inhibitory synaptic terminals. We demonstrate a differential role between types of excitatory terminals. Structural and functional aspects of the hippocampal mossy fiber (MF) synapses were studied in wild-type (WT) mice and in synapsin double-knockout mice (DKO). A severe reduction in the number of synaptic vesicles situated more than 100 nm away from the presynaptic membrane active zone was found in the synapsin DKO animals. The ultrastructural level gave concomitant reduction in F-actin immunoreactivity observed at the periactive endocytic zone of the MF terminals. Frequency facilitation was normal in synapsin DKO mice at low firing rates (approximately 0.1 Hz) but was impaired at firing rates within the physiological range (approximately 2 Hz). Synapses made by associational/commissural fibers showed comparatively small frequency facilitation at the same frequencies. Synapsin-dependent facilitation in MF synapses of WT mice was attenuated by blocking F-actin polymerization with cytochalasin B in hippocampal slices. Synapsin III, selectively seen in MF synapses, is enriched specifically in the area adjacent to the synaptic cleft. This may underlie the ability of synapsin III to promote synaptic depression, contributing to the reduced frequency facilitation observed in the absence of synapsins I and II.
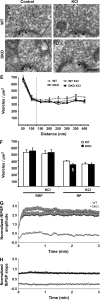
Figures

References
-
- Ashton AC, Ushkaryov YA. Properties of synaptic vesicle pools in mature central nerve terminals. J Biol Chem. 2005;280:37278–37288. - PubMed
-
- Atwood HL, Karunanithi S. Diversification of synaptic strength: presynaptic elements. Nat Rev Neurosci. 2002;3:497–516. - PubMed
-
- Bahler M, Greengard P. Synapsin I bundles F-actin in a phosphorylation-dependent manner. Nature. 1987;326:704–707. - PubMed

