Cytoplasmic cAMP concentrations in intact cardiac myocytes
- PMID: 18550706
- PMCID: PMC2518413
- DOI: 10.1152/ajpcell.00038.2008
Cytoplasmic cAMP concentrations in intact cardiac myocytes
Abstract
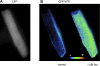
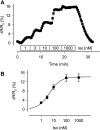
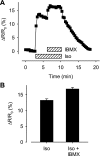
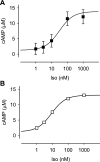
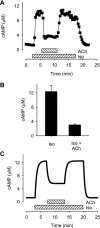
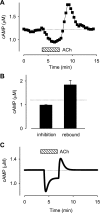
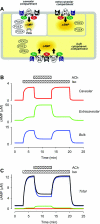
In cardiac myocytes there is evidence that activation of some receptors can regulate protein kinase A (PKA)-dependent responses by stimulating cAMP production that is limited to discrete intracellular domains. We previously developed a computational model of compartmentalized cAMP signaling to investigate the feasibility of this idea. The model was able to reproduce experimental results demonstrating that both beta(1)-adrenergic and M(2) muscarinic receptor-mediated cAMP changes occur in microdomains associated with PKA signaling. However, the model also suggested that the cAMP concentration throughout most of the cell could be significantly higher than that found in PKA-signaling domains. In the present study we tested this counterintuitive hypothesis using a freely diffusible fluorescence resonance energy transfer-based biosensor constructed from the type 2 exchange protein activated by cAMP (Epac2-camps). It was determined that in adult ventricular myocytes the basal cAMP concentration detected by the probe is approximately 1.2 muM, which is high enough to maximally activate PKA. Furthermore, the probe detected responses produced by both beta(1) and M(2) receptor activation. Modeling suggests that responses detected by Epac2-camps mainly reflect what is happening in a bulk cytosolic compartment with little contribution from microdomains where PKA signaling occurs. These results support the conclusion that even though beta(1) and M(2) receptor activation can produce global changes in cAMP, compartmentation plays an important role by maintaining microdomains where cAMP levels are significantly below that found throughout most of the cell. This allows receptor stimulation to regulate cAMP activity over concentration ranges appropriate for modulating both higher (e.g., PKA) and lower affinity (e.g., Epac) effectors.
Figures
References
-
- Adams SR, Harootunian AT, Buechler YJ, Taylor SS, Tsien RY. Fluorescence ratio imaging of cyclic AMP in single cells. Nature 349: 694–697, 1991. - PubMed
-
- Belevych AE, Juranek I, Harvey RD. Protein kinase C regulates functional coupling of β1-adrenergic receptors to Gi/o-mediated responses in cardiac myocytes. FASEB J 18: 367–369, 2004. - PubMed
-
- Bers DM Excitation-Contraction Coupling and Cardiac Contractile Force. Dordrecht, The Netherlands: Kluwer, 2001.
-
- Bos JL EpaC proteins: multi-purpose cAMP targets. Trends Biochem Sci 31: 680–686, 2006. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

