Cell type-specific firing during ripple oscillations in the hippocampal formation of humans
- PMID: 18550752
- PMCID: PMC2693199
- DOI: 10.1523/JNEUROSCI.0437-08.2008
Cell type-specific firing during ripple oscillations in the hippocampal formation of humans
Abstract
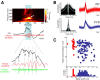
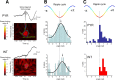
High-frequency field ripples occur in the rodent hippocampal formation and are assumed to depend on interneuron type-specific firing patterns, structuring the activity of pyramidal cells. Ripples with similar characteristics are also present in humans, yet their underlying cellular correlates are still unknown. By in vivo recording interneurons and pyramidal cells in the human hippocampal formation, we find that cell type-specific firing patterns and phase-locking on a millisecond timescale can be distinguished during ripples. In particular, pyramidal cells fired preferentially at the highest amplitude of the ripple, but interneurons began to discharge earlier than pyramidal cells. Furthermore, a large fraction of cells were phase-locked to the ripple cycle, but the preferred phase of discharge of interneurons followed the maximum discharge probability of pyramidal neurons. These relationships between human ripples and unit activity are qualitatively similar to that observed in vivo in the rodents, suggesting that their underlying mechanisms are similar.
Figures

References
-
- Behrens CJ, van den Boom LP, de Hoz L, Friedman A, Heinemann U. Induction of sharp wave-ripple complexes in vitro and reorganization of hippocampal networks. Nat Neurosci. 2005;8:1560–1567. - PubMed
-
- Bragin A, Engel J, Wilson CL, Fried I, Buzsáki G. High-frequency oscillations in human brain. Hippocampus. 1999;9:137–142. - PubMed
-
- Bragin A, Wilson CL, Engel J. Chronic epileptogenesis requires development of a network of pathologically interconnected neuron clusters: a hypothesis. Epilepsia. 2000;41S:144–152. - PubMed
-
- Bragin A, Wilson CL, Staba RJ, Reddick M, Fried I, Engel J. Interictal high-frequency oscillations (80–500 Hz) in the human epileptic brain: entorhinal cortex. Ann Neurol. 2002a;52:407–415. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
