The fold of alpha-synuclein fibrils
- PMID: 18550842
- PMCID: PMC2438424
- DOI: 10.1073/pnas.0712179105
The fold of alpha-synuclein fibrils
Abstract
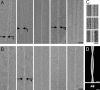
The aggregation of proteins into amyloid fibrils is associated with several neurodegenerative diseases. In Parkinson's disease it is believed that the aggregation of alpha-synuclein (alpha-syn) from monomers by intermediates into amyloid fibrils is the toxic disease-causative mechanism. Here, we studied the structure of alpha-syn in its amyloid state by using various biophysical approaches. Quenched hydrogen/deuterium exchange NMR spectroscopy identified five beta-strands within the fibril core comprising residues 35-96 and solid-state NMR data from amyloid fibrils comprising the fibril core residues 30-110 confirmed the presence of beta-sheet secondary structure. The data suggest that beta1-strand interacts with beta2, beta2 with beta3, beta3 with beta4, and beta4 with beta5. High-resolution cryoelectron microscopy revealed the protofilament boundaries of approximately 2 x 3.5 nm. Based on the combination of these data and published structural studies, a fold of alpha-syn in the fibrils is proposed and discussed.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
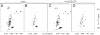


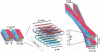
References
-
- Goedert M. Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci. 2001;2:492–501. - PubMed
-
- Cookson MR. The biochemistry of Parkinson's disease. Annu Rev Biochem. 2005;74:29–52. - PubMed
-
- Recchia A, et al. Alpha-synuclein and Parkinson's disease. FASEB J. 2004;18:617–626. - PubMed
-
- Lee VM, Trojanowski JQ. Mechanisms of Parkinson's disease linked to pathological alpha-synuclein: New targets for drug discovery. Neuron. 2006;52:33–38. - PubMed
-
- Hashimoto M, Rockenstein E, Masliah E. Transgenic models of alpha-synuclein pathology: Past, present, and future. Ann NY Acad Sci. 2003;991:171–188. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

