The malaria secretome: from algorithms to essential function in blood stage infection
- PMID: 18551176
- PMCID: PMC2408878
- DOI: 10.1371/journal.ppat.1000084
The malaria secretome: from algorithms to essential function in blood stage infection
Erratum in
- PLoS Pathog. 2008 Jun;4(6). doi: 10.1371/annotation/2b000375-e083-46ed-a44a-ff297e6c37d0. Waters, Andy [corrected to Waters, Andrew P]; Janse, Chris [corrected to Janse, Chris J]
Abstract
The malaria agent Plasmodium falciparum is predicted to export a "secretome" of several hundred proteins to remodel the host erythrocyte. Prediction of protein export is based on the presence of an ER-type signal sequence and a downstream Host-Targeting (HT) motif (which is similar to, but distinct from, the closely related Plasmodium Export Element [PEXEL]). Previous attempts to determine the entire secretome, using either the HT-motif or the PEXEL, have yielded large sets of proteins, which have not been comprehensively tested. We present here an expanded secretome that is optimized for both P. falciparum signal sequences and the HT-motif. From the most conservative of these three secretome predictions, we identify 11 proteins that are preserved across human- and rodent-infecting Plasmodium species. The conservation of these proteins likely indicates that they perform important functions in the interaction with and remodeling of the host erythrocyte important for all Plasmodium parasites. Using the piggyBac transposition system, we validate their export and find a positive prediction rate of approximately 70%. Even for proteins identified by all secretomes, the positive prediction rate is not likely to exceed approximately 75%. Attempted deletions of the genes encoding the conserved exported proteins were not successful, but additional functional analyses revealed the first conserved secretome function. This gave new insight into mechanisms for the assembly of the parasite-induced tubovesicular network needed for import of nutrients into the infected erythrocyte. Thus, genomic screens combined with functional assays provide unexpected and fundamental insights into host remodeling by this major human pathogen.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

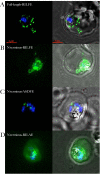
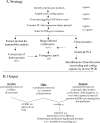
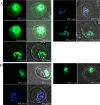
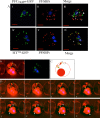

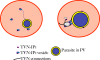
References
-
- Suwanarusk R, Cooke BM, Dondorp AM, Silamut K, Sattabongkot J, et al. The deformability of red blood cells parasitized by Plasmodium falciparum and P. vivax. J Infect Dis. 2004;189:190–194. - PubMed
-
- Desai SA, Bezrukov SM, Zimmerberg J. A voltage-dependent channel involved in nutrient uptake by red blood cells infected with the malaria parasite. Nature. 2000;406:1001–1005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

