Adipocyte/macrophage fatty acid-binding proteins contribute to metabolic deterioration through actions in both macrophages and adipocytes in mice
- PMID: 18551191
- PMCID: PMC2423863
- DOI: 10.1172/JCI34750
Adipocyte/macrophage fatty acid-binding proteins contribute to metabolic deterioration through actions in both macrophages and adipocytes in mice
Abstract
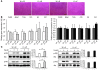
Adipose tissue inflammation is a characteristic of obesity. However, the mechanisms that regulate this inflammatory response and link adipose inflammation to systemic metabolic consequences are not fully understood. In this study, we have taken advantage of the highly restricted coexpression of adipocyte/macrophage fatty acid-binding proteins (FABPs) aP2 (FABP4) and mal1 (FABP5) to examine the contribution of these lipid chaperones in macrophages and adipocytes to local and systemic inflammation and metabolic homeostasis in mice. Deletion of FABPs in adipocytes resulted in reduced expression of inflammatory cytokines in macrophages, whereas the same deletion in macrophages led to enhanced insulin signaling and glucose uptake in adipocytes. Using radiation chimerism through bone marrow transplantation, we generated mice with FABP deficiency in bone marrow and stroma-derived elements in vivo and studied the impact of each cellular target on local and systemic insulin action and glucose metabolism in dietary obesity. The results of these experiments indicated that neither macrophages nor adipocytes individually could account for the total impact of FABPs on systemic metabolism and suggest that interactions between these 2 cell types, particularly in adipose tissue, are critical for the inflammatory basis of metabolic deterioration.
Figures




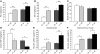

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

