Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug
- PMID: 18551193
- PMCID: PMC2423865
- DOI: 10.1172/JCI35017
Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug
Abstract
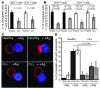
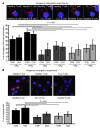
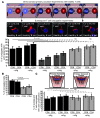
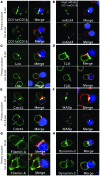
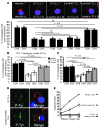
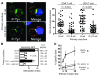
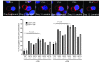
Cancer is associated with immune deficiency, but the biologic basis of this is poorly defined. Here we demonstrate that impaired actin polymerization results in CD4+ and CD8+ T cells from patients with chronic lymphocytic leukemia (CLL) exhibiting defective immunological synapse formation with APCs. Although this synapse dysfunction was in part a result of the CLL cells having poor APC function, defective actin polymerization was also identified in T cells from patients with CLL. We further demonstrate that, following contact with CLL cells, defects in immune synapse formation were induced in healthy allogeneic T cells. This required direct contact and was inhibited by blocking adhesion molecules on CLL B cells. In T cells from patients with CLL and in T cells from healthy individuals that had been in contact with CLL cells, recruitment of key regulatory proteins to the immune synapse was inhibited. Treatment of autologous T cells and CLL cells with the immunomodulating drug lenalidomide resulted in improved synapse formation. These results define what we believe to be a novel immune dysfunction in T cells from patients with CLL that has implications for both autologous and allogeneic immunotherapy approaches and identifies repair of immune synapse defects as an essential step in improving cancer immunotherapy approaches.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

