Fever-induced QTc prolongation and ventricular arrhythmias in individuals with type 2 congenital long QT syndrome
- PMID: 18551196
- PMCID: PMC2423868
- DOI: 10.1172/JCI35337
Fever-induced QTc prolongation and ventricular arrhythmias in individuals with type 2 congenital long QT syndrome
Abstract
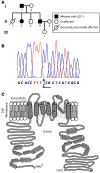
Type 2 congenital long QT syndrome (LQT-2) is linked to mutations in the human ether a-go-go-related gene (HERG) and is characterized by rate-corrected QT interval (QTc) prolongation, ventricular arrhythmias, syncope, and sudden death. Recognized triggers of these cardiac events include emotional and acoustic stimuli. Here we investigated the repeated occurrence of fever-induced polymorphic ventricular tachycardia and ventricular fibrillation in 2 LQT-2 patients with A558P missense mutation in HERG. ECG analysis showed increased QTc with fever in both patients. WT, A558P, and WT+A558P HERG were expressed heterologously in HEK293 cells and were studied using biochemical and electrophysiological techniques. A558P proteins showed a trafficking-deficient phenotype. WT+A558P coexpression caused a dominant-negative effect, selectively accelerated the rate of channel inactivation, and reduced the temperature-dependent increase in the WT current. Thus, the WT+A558P current did not increase to the same extent as the WT current, leading to larger current density differences at higher temperatures. A similar temperature-dependent phenotype was seen for coexpression of the trafficking-deficient LQT-2 F640V mutation. We postulate that the weak increase in the HERG current density in WT-mutant coassembled channels contributes to the development of QTc prolongation and arrhythmias at febrile temperatures and suggest that fever is a potential trigger of life-threatening arrhythmias in LQT-2 patients.
Figures