Nitrite reductase activity of hemoglobin as a systemic nitric oxide generator mechanism to detoxify plasma hemoglobin produced during hemolysis
- PMID: 18552166
- PMCID: PMC2519206
- DOI: 10.1152/ajpheart.00151.2008
Nitrite reductase activity of hemoglobin as a systemic nitric oxide generator mechanism to detoxify plasma hemoglobin produced during hemolysis
Abstract
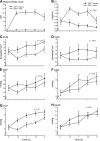
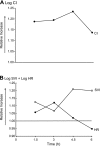
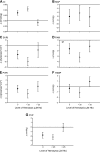
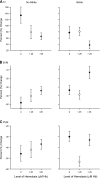
Hemoglobin (Hb) potently inactivates the nitric oxide (NO) radical via a dioxygenation reaction forming nitrate (NO(3)(-)). This inactivation produces endothelial dysfunction during hemolytic conditions and may contribute to the vascular complications of Hb-based blood substitutes. Hb also functions as a nitrite (NO(2)(-)) reductase, converting nitrite into NO as it deoxygenates. We hypothesized that during intravascular hemolysis, nitrite infusions would limit the vasoconstrictive properties of plasma Hb. In a canine model of low- and high-intensity hypotonic intravascular hemolysis, we characterized hemodynamic responses to nitrite infusions. Hemolysis increased systemic and pulmonary arterial pressures and systemic vascular resistance. Hemolysis also inhibited NO-dependent pulmonary and systemic vasodilation by the NO donor sodium nitroprusside. Compared with nitroprusside, nitrite demonstrated unique effects by not only inhibiting hemolysis-associated vasoconstriction but also by potentiating vasodilation at plasma Hb concentrations of <25 muM. We also observed an interaction between plasma Hb levels and nitrite to augment nitroprusside-induced vasodilation of the pulmonary and systemic circulation. This nitrite reductase activity of Hb in vivo was recapitulated in vitro using a mitochondrial NO sensor system. Nitrite infusions may promote NO generation from Hb while maintaining oxygen delivery; this effect could be harnessed to treat hemolytic conditions and to detoxify Hb-based blood substitutes.
Figures

References
-
- Aessopos A, Stamatelos G, Skoumas V, Vassilopoulos G, Mantzourani M, Loukopoulos D. Pulmonary hypertension and right heart failure in patients with beta- thalassemia intermedia. Chest 107: 50–53, 1995. - PubMed
-
- Azarov I, Huang KT, Basu S, Gladwin MT, Hogg N, Kim-Shapiro DB. Nitric oxide scavenging by red blood cells as a function of hematocrit and oxygenation. J Biol Chem 280: 39024–39032, 2005. - PubMed
-
- Basu S, Grubina R, Huang J, Conradie J, Huang Z, Jeffers A, Jiang A, He X, Azarov I, Seibert R, Mehta A, Patel R, King SB, Hogg N, Ghosh A, Gladwin MT, Kim-Shapiro DB. Catalytic generation of N2O3 by the concerted nitrite reductase and anhydrase activity of hemoglobin. Nat Chem Biol 3: 785–794, 2007. - PubMed
-
- Brooks J The action of nitrite on haemoglobin in the absence of oxygen. Proc R Soc Med 123: 368–382, 1937.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

