Lymphoid precursors are directed to produce dendritic cells as a result of TLR9 ligation during herpes infection
- PMID: 18552210
- PMCID: PMC2572801
- DOI: 10.1182/blood-2008-04-151506
Lymphoid precursors are directed to produce dendritic cells as a result of TLR9 ligation during herpes infection
Abstract
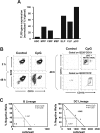
Hematopoietic stem and progenitor cells were previously found to express Toll-like receptors (TLRs), suggesting that bacterial/viral products may influence blood cell formation. We now show that common lymphoid progenitors (CLPs) from mice with active HSV-1 infection are biased to dendritic cell (DC) differentiation, and the phenomenon is largely TLR9 dependent. Similarly, CLPs from mice treated with the TLR9 ligand CpG ODN had little ability to generate CD19+ B lineage cells and had augmented competence to generate DCs. TNFalpha mediates the depletion of late-stage lymphoid progenitors from bone marrow in many inflammatory conditions, but redirection of lymphopoiesis occurred in TNFalpha-/- mice treated with CpG ODN. Increased numbers of DCs with a lymphoid past were identified in Ig gene recombination substrate reporter mice treated with CpG ODN. TLR9 is highly expressed on lymphoid progenitors, and culture studies revealed that those receptors, rather than inflammatory cytokines, accounted for the production of several types of functional DCs. Common myeloid progenitors are normally a good source of DCs, but this potential was reduced by TLR9 ligation. Thus, alternate differentiation pathways may be used to produce innate effector cells in health and disease.
Figures



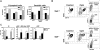
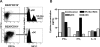
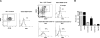
Comment in
-
The hematopoiesis paradigm: clarity or ambiguity?Blood. 2008 Nov 1;112(9):3534-5. doi: 10.1182/blood-2008-07-167759. Blood. 2008. PMID: 18948582 Free PMC article.
References
-
- Hardy RR, Kincade PW, Dorshkind K. The protean nature of cells in the B lymphocyte lineage. Immunity. 2007;26:703–714. - PubMed
-
- Welner RS, Pelayo R, Kincade PW. Evolving views on the genealogy of B cells. Nat Rev Immunol. 2008;8:95–106. - PubMed
-
- Wu L, Liu YJ. Development of dendritic-cell lineages. Immunity. 2007;26:741–750. - PubMed
-
- Fogg DK, Sibon C, Miled C, et al. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 AI069024/AI/NIAID NIH HHS/United States
- R01 AI050737/AI/NIAID NIH HHS/United States
- R03 AR054529/AR/NIAMS NIH HHS/United States
- AI58162/AI/NIAID NIH HHS/United States
- AI50737/AI/NIAID NIH HHS/United States
- T32 AI007633/AI/NIAID NIH HHS/United States
- R01 AI020069/AI/NIAID NIH HHS/United States
- AI053108/AI/NIAID NIH HHS/United States
- R01 AI053108/AI/NIAID NIH HHS/United States
- AI20069/AI/NIAID NIH HHS/United States
- AR054529/AR/NIAMS NIH HHS/United States
- R21 AI050737/AI/NIAID NIH HHS/United States
- AI069024/AI/NIAID NIH HHS/United States
- R01 AI058162/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

