Drosophila RalA is essential for the maintenance of Jak/Stat signalling in ovarian follicles
- PMID: 18552769
- PMCID: PMC2475328
- DOI: 10.1038/embor.2008.79
Drosophila RalA is essential for the maintenance of Jak/Stat signalling in ovarian follicles
Abstract
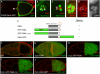
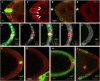
Small GTPases of the Ras-like (Ral) family are crucial for signalling functions in both normal and cancer cells; however, their role in a developing organism is poorly understood. Here, we identify the Drosophila Ral homologue RalA as a new key regulator of polar-cell differentiation during oogenesis. Polar cells have a crucial role in patterning the egg chamber and in recruiting border cells, which undergo collective and guided migration. We show that RalA function is essential for the maintenance of anterior and posterior polar-cell fate and survival. RalA is required cell autonomously to control the expression of polar-cell-specific markers, including the Jak/Stat ligand Unpaired. The loss of RalA also causes a cell non-autonomous phenotype owing to reduced Jak/Stat signalling in neighbouring follicle cells. As a result, border-cell assembly and migration as well as the polarization of the oocyte are defective. Thus, RalA is required in organizing centres to control proper patterning and migration in vivo.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

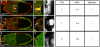

References
-
- Beccari S, Teixeira L, Rorth P (2002) The JAK/STAT pathway is required for border cell migration during Drosophila oogenesis. Mech Dev 111: 115–123 - PubMed
-
- Besse F, Pret AM (2003) Apoptosis-mediated cell death within the ovarian polar cell lineage of Drosophila melanogaster. Development 130: 1017–1027 - PubMed
-
- Bianco A, Poukkula M, Cliffe A, Mathieu J, Luque CM, Fulga TA, Rørth P (2007) Two distinct modes of guidance signalling during collective migration of border cells. Nature 448: 362–365 - PubMed
-
- Bourbon HM et al. (2002) A P-insertion screen identifying novel X-linked essential genes in Drosophila. Mech Dev 110: 71–83 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

