A tumor necrosis factor-alpha-mediated pathway promoting autosomal dominant polycystic kidney disease
- PMID: 18552856
- PMCID: PMC3359869
- DOI: 10.1038/nm1783
A tumor necrosis factor-alpha-mediated pathway promoting autosomal dominant polycystic kidney disease
Abstract
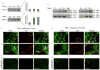
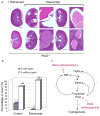
Autosomal dominant polycystic kidney disease (ADPKD) is caused by heterozygous mutations in either PKD1 or PKD2, genes that encode polycystin-1 and polycystin-2, respectively. We show here that tumor necrosis factor-alpha (TNF-alpha), an inflammatory cytokine present in the cystic fluid of humans with ADPKD, disrupts the localization of polycystin-2 to the plasma membrane and primary cilia through a scaffold protein, FIP2, which is induced by TNF-alpha. Treatment of mouse embryonic kidney organ cultures with TNF-alpha resulted in formation of cysts, and this effect was exacerbated in the Pkd2(+/-) kidneys. TNF-alpha also stimulated cyst formation in vivo in Pkd2(+/-) mice. In contrast, treatment of Pkd2(+/-) mice with the TNF-alpha inhibitor etanercept prevented cyst formation. These data reveal a pathway connecting TNF-alpha signaling, polycystins and cystogenesis, the activation of which may reduce functional polycystin-2 below a critical threshold, precipitating the ADPKD cellular phenotype.
Figures

References
-
- Wilson PD. Polycystic kidney disease. The New England journal of medicine. 2004;350:151–64. - PubMed
-
- Qian F, Watnick TJ, Onuchic LF, Germino GG. The molecular basis of focal cyst formation in human autosomal dominant polycystic kidney disease type I. Cell. 1996;87:979–87. - PubMed
-
- Lantinga-van Leeuwen IS, et al. Lowering of Pkd1 expression is sufficient to cause polycystic kidney disease. Hum Mol Genet. 2004;13:3069–77. - PubMed
-
- Martinez JR, Grantham JJ. Polycystic kidney disease: etiology, pathogenesis, and treatment. Dis Mon. 1995;41:693–765. - PubMed
-
- Pfeffer K. Biological functions of tumor necrosis factor cytokines and their receptors. Cytokine Growth Factor Rev. 2003;14:185–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

