Dose-response curve slope sets class-specific limits on inhibitory potential of anti-HIV drugs
- PMID: 18552857
- PMCID: PMC2743464
- DOI: 10.1038/nm1777
Dose-response curve slope sets class-specific limits on inhibitory potential of anti-HIV drugs
Abstract
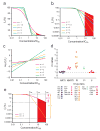
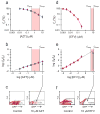
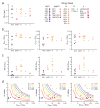
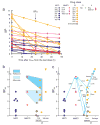
Highly active antiretroviral therapy (HAART) can control HIV-1 replication, but suboptimal treatment allows for the evolution of resistance and rebound viremia. A comparative measure of antiviral activity under clinically relevant conditions would guide drug development and the selection of regimens that maximally suppress replication. Here we show that current measures of antiviral activity, including IC(50) and inhibitory quotient, neglect a key dimension, the dose-response curve slope. Using infectivity assays with wide dynamic range, we show that this slope has noteworthy effects on antiviral activity. Slope values are class specific for antiviral drugs and define intrinsic limitations on antiviral activity for some classes. Nucleoside reverse transcriptase inhibitors and integrase inhibitors have slopes of approximately 1, characteristic of noncooperative reactions, whereas non-nucleoside reverse transcriptase inhibitors, protease inhibitors and fusion inhibitors unexpectedly show slopes >1. Instantaneous inhibitory potential (IIP), the log reduction in single-round infectivity at clinical drug concentrations, is strongly influenced by slope and varies by >8 logs for anti-HIV drugs. IIP provides a more accurate measure of antiviral activity and in general correlates with clinical outcomes. Only agents with slopes >1 achieve high-level inhibition of single-round infectivity, a finding with profound implications for drug and vaccine development.
Figures
References
-
- Gulick RM, et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337:734–739. - PubMed
-
- Hammer SM, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337:725–733. - PubMed
-
- Ho DD, et al. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. - PubMed
-
- Wei X, et al. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–122. - PubMed
-
- Preston BD, Poiesz BJ, Loeb LA. Fidelity of HIV-1 reverse transcriptase. Science. 1988;242:1168–1171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

