Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded
- PMID: 18552863
- PMCID: PMC3947522
- DOI: 10.1038/nrmicro1927
Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded
Abstract
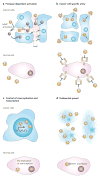
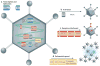
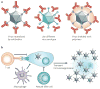
Virotherapy is currently undergoing a renaissance, based on our improved understanding of virus biology and genetics and our better knowledge of many different types of cancer. Viruses can be reprogrammed into oncolytic vectors by combining three types of modification: targeting, arming and shielding. Targeting introduces multiple layers of cancer specificity and improves safety and efficacy; arming occurs through the expression of prodrug convertases and cytokines; and coating with polymers and the sequential usage of different envelopes or capsids provides shielding from the host immune response. Virus-based therapeutics are beginning to find their place in cancer clinical practice, in combination with chemotherapy and radiation.
Figures


References
-
- Dock G. The influence of complicating diseases upon leukemia. Am J Med Sci. 1904;127:563–592.
-
- Sinkovics J, Horvath J. New developments in the virus therapy of cancer: a historical review. Intervirology. 1993;36:193–214. - PubMed
-
- Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol Ther. 2007;15:651–659. A compelling narrative of the first 100 years of oncolytic viruses. - PubMed
-
- Hoster H, Zanes R, Vonhaam E. The association of “viral” hepatitis and Hodgkin’s disease. Cancer Res. 1949;9:473–480. - PubMed
-
- Moore AE. Viruses with oncolytic properties and their adaptation to tumors. Ann NY Acad Sci. 1952;54:945–952. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

