Prediction of clinical response based on pharmacokinetic/pharmacodynamic models of 5-hydroxytryptamine reuptake inhibitors in mice
- PMID: 18552871
- PMCID: PMC2538695
- DOI: 10.1038/bjp.2008.243
Prediction of clinical response based on pharmacokinetic/pharmacodynamic models of 5-hydroxytryptamine reuptake inhibitors in mice
Abstract
Background and purpose: Bridging the gap between preclinical research and clinical trials is vital for drug development. Predicting clinically relevant steady-state drug concentrations (Css) in serum from preclinical animal models may facilitate this transition. Here we used a pharmacokinetic/pharmacodynamic (PK/PD) modelling approach to evaluate the predictive validity of 5-hydroxytryptamine (5-HT; serotonin) transporter (SERT) occupancy and 5-hydroxytryptophan (5-HTP)-potentiated behavioral syndrome induced by 5-HT reuptake inhibitor (SRI) antidepressants in mice.
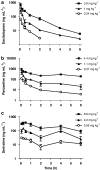
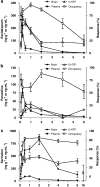
Experimental approach: Serum and whole brain drug concentrations, cortical SERT occupancy and 5-HTP-potentiated behavioral syndrome were measured over 6 h after a single subcutaneous injection of escitalopram, paroxetine or sertraline. [(3)H]2-(2-dimethylaminomethylphenylsulphanyl)-5-methyl-phenylamine ([(3)H]MADAM) was used to assess SERT occupancy. For PK/PD modelling, an effect-compartment model was applied to collapse the hysteresis and predict the steady-state relationship between drug exposure and PD response.
Key results: The predicted Css for escitalopram, paroxetine and sertraline at 80% SERT occupancy in mice are 18 ng mL(-1), 18 ng mL(-1) and 24 ng mL(-1), respectively, with corresponding responses in the 5-HTP behavioral model being between 20-40% of the maximum.
Conclusions and implications: Therapeutically effective SERT occupancy for SRIs in depressed patients is approximately 80%, and the corresponding plasma Css are 6-21 ng mL(-1), 21-95 ng mL(-1) and 20-48 ng mL(-1) for escitalopram, paroxetine and sertraline, respectively. Thus, PK/PD modelling using SERT occupancy and 5-HTP-potentiated behavioral syndrome as response markers in mice may be a useful tool to predict clinically relevant plasma Css values.
Figures



Similar articles
-
A comparative review of escitalopram, paroxetine, and sertraline: Are they all alike?Int Clin Psychopharmacol. 2014 Jul;29(4):185-96. doi: 10.1097/YIC.0000000000000023. Int Clin Psychopharmacol. 2014. PMID: 24424469 Free PMC article. Review.
-
Relationship between brain serotonin transporter binding, plasma concentration and behavioural effect of selective serotonin reuptake inhibitors.Br J Pharmacol. 2005 Mar;144(5):695-702. doi: 10.1038/sj.bjp.0706108. Br J Pharmacol. 2005. PMID: 15678084 Free PMC article.
-
In vivo imaging of serotonin transporter occupancy by means of SPECT and [123I]ADAM in healthy subjects administered different doses of escitalopram or citalopram.Psychopharmacology (Berl). 2006 Oct;188(3):263-72. doi: 10.1007/s00213-006-0486-0. Epub 2006 Sep 6. Psychopharmacology (Berl). 2006. PMID: 16955282 Clinical Trial.
-
Higher serotonin transporter occupancy after multiple dose administration of escitalopram compared to citalopram: an [123I]ADAM SPECT study.Psychopharmacology (Berl). 2007 Apr;191(2):333-9. doi: 10.1007/s00213-006-0666-y. Epub 2007 Jan 19. Psychopharmacology (Berl). 2007. PMID: 17235610 Clinical Trial.
-
[Escitalopram: a selective inhibitor and allosteric modulator of the serotonin transporter].Encephale. 2007 Dec;33(6):965-72. doi: 10.1016/j.encep.2007.11.001. Epub 2007 Dec 11. Encephale. 2007. PMID: 18789789 Review. French.
Cited by
-
Continuous home cage monitoring of activity and sleep in mice during repeated paroxetine treatment and discontinuation.Psychopharmacology (Berl). 2023 Nov;240(11):2403-2418. doi: 10.1007/s00213-023-06442-3. Epub 2023 Aug 16. Psychopharmacology (Berl). 2023. PMID: 37584734 Free PMC article.
-
Encapsulated Escitalopram and Paroxetine Intranasal Co-Administration: In Vitro/In Vivo Evaluation.Front Pharmacol. 2021 Dec 2;12:751321. doi: 10.3389/fphar.2021.751321. eCollection 2021. Front Pharmacol. 2021. PMID: 34925013 Free PMC article.
-
Escitalopram facilitates tumor growth and metastasis in rodents: Is it safe?Neoplasia. 2025 Aug;66:101182. doi: 10.1016/j.neo.2025.101182. Epub 2025 May 23. Neoplasia. 2025. PMID: 40411973 Free PMC article.
-
Modulation of serotonin transporter expression by escitalopram under inflammation.Commun Biol. 2024 Jun 8;7(1):710. doi: 10.1038/s42003-024-06240-3. Commun Biol. 2024. PMID: 38851804 Free PMC article.
-
Antidepressant response to chronic citalopram treatment in eight inbred mouse strains.Psychopharmacology (Berl). 2011 Feb;213(2-3):509-20. doi: 10.1007/s00213-010-2140-0. Epub 2010 Dec 22. Psychopharmacology (Berl). 2011. PMID: 21181117
References
-
- American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders 2000. 4th edn, American Psychiatric Press, Inc: Washington
-
- Asberg M, Eriksson B, Martensson B, Traskman-Bendz L, Wagner A. Therapeutic effects of serotonin uptake inhibitors in depression. J Clin Psychiatry. 1986;47:23–35. - PubMed
-
- Baumann P. Pharmacokinetic–pharmacodynamic relationship of the selective serotonin reuptake inhibitors. Clin Pharmacokinet. 1996;31:444–469. - PubMed
-
- Bech P, Tanghoj P, Andersen HF, Overo K. Citalopram dose–response revisited using an alternative psychometric approach to evaluate clinical effects of four fixed citalopram doses compared to placebo in patients with major depression. Psychopharmacology (Berl) 2002;163:20–25. - PubMed
-
- Bech P, Tanghoj P, Cialdella P, Andersen HF, Pedersen AG. Escitalopram dose–response revisited: an alternative psychometric approach to evaluate clinical effects of escitalopram compared to citalopram and placebo in patients with major depression. Int J Neuropsychopharmacol. 2004;7:283–290. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

