Vitamin D and glucocorticoids differentially modulate chemokine expression in human airway smooth muscle cells
- PMID: 18552877
- PMCID: PMC2440089
- DOI: 10.1038/bjp.2008.232
Vitamin D and glucocorticoids differentially modulate chemokine expression in human airway smooth muscle cells
Abstract
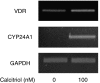
Background and purpose: Chemokines play a critical role in the pathogenesis of asthma and facilitate the recruitment of inflammatory cells in the airways. Evidence now suggests that airway smooth muscle (ASM) may serve as a source of chemokines in inflamed airways. Although vitamin D has potent anti-inflammatory properties in vitro in some cell types, its effects on ASM cells remain unclear. Here, we investigated whether 1alpha, 25-dihydroxy vitamin D3 (calcitriol) modulated chemokine production in ASM.
Experimental approach: Human ASM cell cultures were derived from tracheal samples taken during surgery. ASM cells were treated with tumour necrosis factor alpha (TNFalpha) and/or interferon gamma (IFNgamma) for 24 h in the presence of calcitriol and/or the glucocorticoid fluticasone added 2 h before. RANTES (regulated upon activation, normal T-cell expressed and secreted), interferon-inducible protein 10 (IP-10) and fractalkine (FKN) levels in cell supernatants were measured by ELISA.
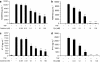
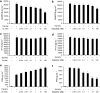
Key results: In TNFalpha-treated cells, calcitriol inhibited RANTES and IP-10 secretion in a concentration-dependent manner. FKN levels were negligible. In TNFalpha/IFNgamma-treated cells, whereas fluticasone or calcitriol alone partially inhibited RANTES secretion (by 38 and 20%, respectively), the combination of both drugs additively inhibited RANTES secretion (by 60%). No effect was observed on IP-10 secretion. Whereas fluticasone enhanced FKN secretion (by 50%), calcitriol significantly decreased FKN levels (by 50%). Interestingly, calcitriol blocked the stimulatory effect of fluticasone on FKN secretion, which was inhibited by 60% with the combination of calcitriol and fluticasone.
Conclusions and implications: These findings suggest that vitamin D uniquely modulates human ASM expression of chemokines and may exert some beneficial effects in the treatment of steroid-resistant patients with asthma.
Figures
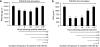

References
-
- Adcock IM, Lane SJ. Corticosteroid-insensitive asthma: molecular mechanisms. J Endocrinol. 2003;178:347–355. - PubMed
-
- Barnes PJ, Hansel TT. Prospects for new drugs for chronic obstructive pulmonary disease. Lancet. 2004;364:985–996. - PubMed
-
- Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin D and pulmonary function in the third National Health and Nutrition Examination survey. Chest. 2005;128:3792–3798. - PubMed
-
- Bosse Y, Maghni K, Hudson TJ. 1Alpha,25-dihydroxy-vitamin D3 stimulation of bronchial smooth muscle cells induces autocrine, contractility, and remodeling processes. Physiol Genomics. 2007;29:161–168. - PubMed
-
- Brightling CE, Ammit AJ, Kaur D, Black JL, Wardlaw AJ, Hughes JM, et al. The CXCL10/CXCR3 axis mediates human lung mast cell migration to asthmatic airway smooth muscle. Am J Respir Crit Care Med. 2005;171:1103–1108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

