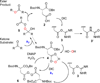
A nonenzymatic acid/peracid catalytic cycle for the Baeyer-Villiger oxidation
- PMID: 18553911
- PMCID: PMC4125978
- DOI: 10.1021/ol8010248
A nonenzymatic acid/peracid catalytic cycle for the Baeyer-Villiger oxidation
Abstract
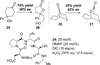
The combined action of carbodiimide and hydrogen peroxide upon exposure to carboxylic acid catalysts serves to generate transient peracids that can be engaged in the Baeyer-Villiger rearrangement of ketones to lactones. Up to 35 turnovers of the catalytic cycle may be achieved. The conditions are especially useful in the context of reactive cyclohexanones, and allow the use of H2O2 as the terminal oxidant. A singular example of a chiral catalyst demonstrates, in principle, that enantioselective catalysis will be possible with this strategy for catalyst turnover.
Figures
References
-
- Baeyer A, Villiger V. Ber. Dtsch. Chem. Ges. 1899;32:3625.
- Krow GR. Org. React. 1993;43:251.
- ten Brink G–J, Arends IWCE, Sheldon RA. Chem. Rev. 2004;104:4105. - PubMed
-
- Mihovilovic MD. Curr. Org. Chem. 2006;10:1265.
- Katsuki T. Russ. Chem. Bull. Int. Ed. 2004;53:1859.
-
- Mihovilovic MD, Muller B, Stanetty P. Eur. J. Org. Chem. 2002;22:3711.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources