Characterization of a streptococcal cholesterol-dependent cytolysin with a lewis y and b specific lectin domain
- PMID: 18553932
- PMCID: PMC2622431
- DOI: 10.1021/bi8005835
Characterization of a streptococcal cholesterol-dependent cytolysin with a lewis y and b specific lectin domain
Abstract
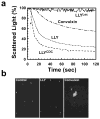
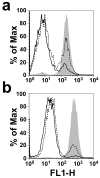
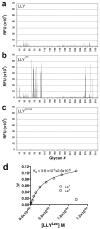
The cholesterol-dependent cytolysins (CDCs) are a large family of pore-forming toxins that often exhibit distinct structural changes that modify their pore-forming activity. A soluble platelet aggregation factor from Streptococcus mitis (Sm-hPAF) was characterized and shown to be a functional CDC with an amino-terminal fucose-binding lectin domain. Sm-hPAF, or lectinolysin (LLY) as renamed herein, is most closely related to CDCs from Streptococcus intermedius (ILY) and Streptococcus pneumoniae (pneumolysin or PLY). The LLY gene was identified in strains of S. mitis, S. pneumoniae, and Streptococcus pseudopneumoniae. LLY induces pore-dependent changes in the light scattering properties of the platelets that mimic those induced by platelet aggregation but does not induce platelet aggregation. LLY monomers form the typical large homooligomeric membrane pore complex observed for the CDCs. The pore-forming activity of LLY on platelets is modulated by the amino-terminal lectin domain, a structure that is not present in other CDCs. Glycan microarray analysis showed the lectin domain is specific for difucosylated glycans within Lewis b (Le (b)) and Lewis y (Le (y)) antigens. The glycan-binding site is occluded in the soluble monomer of LLY but is apparently exposed after cell binding, since it significantly increases LLY pore-forming activity in a glycan-dependent manner. Hence, LLY represents a new class of CDC whose pore-forming mechanism is modulated by a glycan-binding domain.
Figures



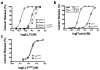

References
-
- Kennedy MJ, Jackson MA, Kearns GL. Delayed diagnosis of penicillin-resistant Streptococcus mitis endocarditis following single-dose amoxicillin prophylaxis in a child. Clin Pediatr (Philadelphia) 2004;43:773–776. - PubMed
-
- Gowda RM, Ansari AW, Khan IA. Complete endocardial cushion defect (complete atrioventricular canal) manifested in adult life by Streptococcus mitis endocarditis of the common atrioventricular valve. Int J Cardiol. 2003;89:109–110. - PubMed
-
- Hall GE, Baddour LM. Apparent failure of endocarditis prophylaxis caused by penicillin-resistant Streptococcus mitis. Am J Med Sci. 2002;324:51–53. - PubMed
-
- Huang IF, Chiou CC, Liu YC, Hsieh KS. Endocarditis caused by penicillin-resistant Streptococcus mitis in a 12-year-old boy, J. Microbiol. Immunol, Infect (China) 2002;35:129–132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

