Kallikrein-modified mesenchymal stem cell implantation provides enhanced protection against acute ischemic kidney injury by inhibiting apoptosis and inflammation
- PMID: 18554097
- PMCID: PMC2743018
- DOI: 10.1089/hum.2008.016
Kallikrein-modified mesenchymal stem cell implantation provides enhanced protection against acute ischemic kidney injury by inhibiting apoptosis and inflammation
Abstract
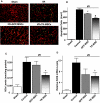
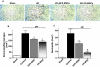
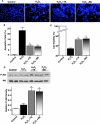
Mesenchymal stem cells (MSCs) migrate to sites of tissue injury and serve as an ideal vehicle for cellular gene transfer. As tissue kallikrein has pleiotropic effects in protection against oxidative organ damage, we investigated the potential of kallikrein-modified MSCs (TK-MSCs) in healing injured kidney after acute ischemia/reperfusion (I/R). TK-MSCs secreted recombinant human kallikrein with elevated vascular endothelial growth factor levels in culture medium, and were more resistant to oxidative stress-induced apoptosis than control MSCs. Expression of human kallikrein was identified in rat glomeruli after I/R injury and systemic TK-MSC injection. Engrafted TK-MSCs exhibited advanced protection against renal injury by reducing blood urea nitrogen, serum creatinine levels, and tubular injury. Six hours after I/R, TK-MSC implantation significantly reduced renal cell apoptosis in association with decreased inducible nitric oxide synthase expression and nitric oxide levels. Forty-eight hours after I/R, TK-MSCs inhibited interstitial neutrophil and monocyte/macrophage infiltration and decreased myeloperoxidase activity, superoxide formation, p38 mitogen-activated protein kinase phosphorylation, and expression of tumor necrosis factor-alpha, monocyte chemoattractant protein-1, and intercellular adhesion molecule-1. In addition, tissue kallikrein and kinin significantly inhibited H2O2-induced apoptosis and increased Akt phosphorylation and cell viability in cultured proximal tubular cells. These results indicate that implantation of kallikrein-modified MSCs in the kidney provides advanced benefits in protection against ischemia-induced kidney injury by suppression of apoptosis and inflammation.
Figures




Similar articles
-
Tissue kallikrein-modified mesenchymal stem cells provide enhanced protection against ischemic cardiac injury after myocardial infarction.Circ J. 2013;77(8):2134-44. doi: 10.1253/circj.cj-12-1585. Epub 2013 May 21. Circ J. 2013. PMID: 23697984
-
14S,21R-dihydroxy-docosahexaenoic acid treatment enhances mesenchymal stem cell amelioration of renal ischemia/reperfusion injury.Stem Cells Dev. 2012 May 1;21(7):1187-99. doi: 10.1089/scd.2011.0220. Epub 2011 Oct 3. Stem Cells Dev. 2012. PMID: 21846180 Free PMC article.
-
Nitric oxide mediates cardiac protection of tissue kallikrein by reducing inflammation and ventricular remodeling after myocardial ischemia/reperfusion.Life Sci. 2008 Jan 16;82(3-4):156-65. doi: 10.1016/j.lfs.2007.10.021. Epub 2007 Nov 9. Life Sci. 2008. PMID: 18068196 Free PMC article.
-
Kallikrein-kinin in stroke, cardiovascular and renal disease.Exp Physiol. 2005 May;90(3):291-8. doi: 10.1113/expphysiol.2004.028464. Epub 2005 Jan 14. Exp Physiol. 2005. PMID: 15653716 Review.
-
Experimental therapy with tissue kallikrein against cerebral ischemia.Front Biosci. 2006 May 1;11:1323-7. doi: 10.2741/1886. Front Biosci. 2006. PMID: 16368519 Review.
Cited by
-
Stem/progenitor cell in kidney: characteristics, homing, coordination, and maintenance.Stem Cell Res Ther. 2021 Mar 20;12(1):197. doi: 10.1186/s13287-021-02266-0. Stem Cell Res Ther. 2021. PMID: 33743826 Free PMC article. Review.
-
Blockade of endogenous tissue kallikrein aggravates renal injury by enhancing oxidative stress and inhibiting matrix degradation.Am J Physiol Renal Physiol. 2010 Apr;298(4):F1033-40. doi: 10.1152/ajprenal.00518.2009. Epub 2010 Jan 20. Am J Physiol Renal Physiol. 2010. PMID: 20089675 Free PMC article.
-
Glutathione S-transferase Mu 2-transduced mesenchymal stem cells ameliorated anti-glomerular basement membrane antibody-induced glomerulonephritis by inhibiting oxidation and inflammation.Stem Cell Res Ther. 2014 Jan 30;5(1):19. doi: 10.1186/scrt408. Stem Cell Res Ther. 2014. PMID: 24480247 Free PMC article.
-
Erythropoietin gene-enhanced marrow mesenchymal stromal cells decrease cisplatin-induced kidney injury and improve survival of allogeneic mice.Mol Ther. 2011 Nov;19(11):2072-83. doi: 10.1038/mt.2011.162. Epub 2011 Aug 16. Mol Ther. 2011. PMID: 21847101 Free PMC article.
-
Kallikrein-kinin in stem cell therapy.World J Stem Cells. 2014 Sep 26;6(4):448-57. doi: 10.4252/wjsc.v6.i4.448. World J Stem Cells. 2014. PMID: 25258666 Free PMC article. Review.
References
-
- Bledsoe G. Shen B. Yao Y. Zhang J.J. Chao L. Chao J. Reversal of renal fibrosis, inflammation, and glomerular hypertrophy by kallikrein gene delivery. Hum. Gene Ther. 2006;17:545–555. - PubMed
-
- Chao J. Chao L. Tillman D.M. Woodley C.M. Margolius H.S. Characterization of monoclonal and polyclonal antibodies to human tissue kallikrein. Hypertension. 1985;7:931–937. - PubMed
-
- Chao J. Zhang J.J. Lin K.F. Chao L. Human kallikrein gene delivery attenuates hypertension, cardiac hypertrophy, and renal injury in Dahl salt-sensitive rats. Hum. Gene Ther. 1998;9:21–31. - PubMed
-
- Chao J. Li H.J. Yao Y.Y. Shen B. Gao L. Bledsoe G. Chao L. Kinin infusion prevents renal inflammation, apoptosis, and fibrosis via inhibition of oxidative stress and mitogen-activated protein kinase activity. Hypertension. 2007;49:490–497. - PubMed
-
- Chatterjee P.K. Patel N.S. Sivarajah A. Kvale E.O. Dugo L. Cuzzocrea S. Brown P.A. Stewart K.N. Mota-Filipe H. Britti D. Yaqoob M.M. Thiemermann C. GW274150, a potent and highly selective inhibitor of iNOS, reduces experimental renal ischemia/reperfusion injury. Kidney Int. 2003;63:853–865. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

