Endogenous anti-inflammatory neuropeptides and pro-resolving lipid mediators: a new therapeutic approach for immune disorders
- PMID: 18554314
- PMCID: PMC4506154
- DOI: 10.1111/j.1582-4934.2008.00387.x
Endogenous anti-inflammatory neuropeptides and pro-resolving lipid mediators: a new therapeutic approach for immune disorders
Abstract
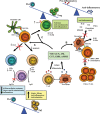
Identification of the factors that regulate the immune tolerance and control the appearance of exacerbated inflammatory conditions is crucial for the development of new therapies of inflammatory and autoimmune diseases. Although much is known about the molecular basis of initiating signals and pro-inflammatory chemical mediators in inflammation, it has only recently become apparent that endogenous stop signals are critical at early checkpoints within the temporal events of inflammation. Some neuropeptides and lipid mediators that are produced during the ongoing inflammatory response have emerged as endogenous anti-inflammatory agents that participate in the regulation of the processes that ensure self-tolerance and/or inflammation resolution. Here we examine the latest research findings, which indicate that neuropeptides participate in maintaining immune tolerance in two distinct ways: by regulating the balance between pro-inflammatory and anti-inflammatory factors, and by inducing the emergence of regulatory T cells with suppressive activity against autoreactive T-cell effectors. On the other hand, we also focus on lipid mediators biosynthesized from omega-3 and omega-6 polyunsaturated fatty-acids in inflammatory exudates that promote the resolution phase of acute inflammation by regulating leucocyte influx to and efflux from local inflamed sites. Both anti-inflammatory neuropeptides and pro-resolving lipid mediators have shown therapeutic potential for a variety of inflammatory and autoimmune disorders and could be used as biotemplates for the development of novel pharmacologic agents.
Figures


References
-
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–61. - PubMed
-
- Goodnow CC. Multistep pathogenesis of autoimmune diseases. Cell. 2007;130:25–35. - PubMed
-
- Nathan C. Points of control in inflammation. Nature. 2002;420:846–52. - PubMed
-
- Mills KHG. Regulatory T cells: friend or foe in immunity to infection? Nat Rev Immunol. 2004;4:841–55. - PubMed
-
- Bluestone JA. Regulatory T-cell therapy: is it ready for the clinic? Nat Rev Immunol. 2005;5:343–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

