Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle
- PMID: 18556367
- PMCID: PMC2538832
- DOI: 10.1113/jphysiol.2008.153916
Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle
Abstract
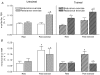
Resistance (RE) and endurance (EE) exercise stimulate mixed skeletal muscle protein synthesis. The phenotypes induced by RE (myofibrillar protein accretion) and EE (mitochondrial expansion) training must result from differential stimulation of myofibrillar and mitochondrial protein synthesis. We measured the synthetic rates of myofibrillar and mitochondrial proteins and the activation of signalling proteins (Akt-mTOR-p70S6K) at rest and after an acute bout of RE or EE in the untrained state and after 10 weeks of RE or EE training in young healthy men. While untrained, RE stimulated both myofibrillar and mitochondrial protein synthesis, 67% and 69% (P < 0.02), respectively. After training, only myofibrillar protein synthesis increased with RE (36%, P = 0.05). EE stimulated mitochondrial protein synthesis in both the untrained, 154%, and trained, 105% (both P < 0.05), but not myofibrillar protein synthesis. Acute RE and EE increased the phosphorylation of proteins in the Akt-mTOR-p70S6K pathway with comparatively minor differences between two exercise stimuli. Phosphorylation of Akt-mTOR-p70S6K proteins was increased after 10 weeks of RE training but not by EE training. Chronic RE or EE training modifies the protein synthetic response of functional protein fractions, with a shift toward exercise phenotype-specific responses, without an obvious explanatory change in the phosphorylation of regulatory signalling pathway proteins.
Figures






References
-
- Atherton PJ, Babraj J, Smith K, Singh J, Rennie MJ, Wackerhage H. Selective activation of AMPK-PGC-1α or PKB-TSC2-mTOR signaling can explain specific adaptive responses to endurance or resistance training-like electrical muscle stimulation. FASEB J. 2005;19:786–788. - PubMed
-
- Balagopal P, Schimke JC, Ades P, Adey D, Nair KS. Age effect on transcript levels and synthesis rate of muscle MHC and response to resistance exercise. Am J Physiol Endocrinol Metab. 2001;280:E203–E208. - PubMed
-
- Beelen M, Koopman R, Gijsen AP, Vandereyt H, Kies AK, Kuipers H, Saris WH, van Loon LJ. Protein co-ingestion stimulates muscle protein synthesis during resistance type exercise. Am J Physiol Endocrinol Metab. 2008 in press. - PubMed
-
- Bezaire V, Heigenhauser GJ, Spriet LL. Regulation of CPT I activity in intermyofibrillar and subsarcolemmal mitochondria from human and rat skeletal muscle. Am J Physiol Endocrinol Metab. 2004;286:E85–E91. - PubMed
-
- Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol Endocrinol Metab. 1995;268:E514–E520. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/X510697/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- RR00954/RR/NCRR NIH HHS/United States
- R01 DK059531/DK/NIDDK NIH HHS/United States
- DK20579/DK/NIDDK NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- BB/C516779/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- DK74345/DK/NIDDK NIH HHS/United States
- DK59531/DK/NIDDK NIH HHS/United States
- P41 RR000954/RR/NCRR NIH HHS/United States
- DK56341/DK/NIDDK NIH HHS/United States
- R01 DK049393/DK/NIDDK NIH HHS/United States
- R21 DK074345/DK/NIDDK NIH HHS/United States
- DK49393/DK/NIDDK NIH HHS/United States
- P60 DK020579/DK/NIDDK NIH HHS/United States
- R56 DK049393/DK/NIDDK NIH HHS/United States
- P30 DK020579/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

