Hepatic Mrp4 induction following acetaminophen exposure is dependent on Kupffer cell function
- PMID: 18556419
- PMCID: PMC2519859
- DOI: 10.1152/ajpgi.00541.2007
Hepatic Mrp4 induction following acetaminophen exposure is dependent on Kupffer cell function
Abstract
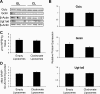
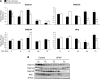
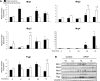
During acetaminophen (APAP) hepatotoxicity, increased expression of multidrug resistance-associated proteins 2, 3, and 4 (Mrp2-4) occurs. Mrp4 is the most significantly upregulated transporter in mouse liver following APAP treatment. Although the expression profiles of liver transporters following APAP hepatotoxicity are well characterized, the regulatory mechanisms contributing to these changes remain unknown. We hypothesized that Kupffer cell-derived mediators participate in the regulation of hepatic transporters during APAP toxicity. To investigate this, C57BL/6J mice were pretreated with clodronate liposomes (0.1 ml iv) to deplete Kupffer cells and then challenged with APAP (500 mg/kg ip). Liver injury was assessed by plasma alanine aminotransferase and hepatic transporter protein expression was determined by Western blot and immunohistochemistry. Depletion of Kupffer cells by liposomal clodronate increased susceptibility to APAP hepatotoxicity. Although increased expression of several efflux transporters was observed after APAP exposure, only Mrp4 was found to be differentially regulated following Kupffer cell depletion. At 48 and 72 h after APAP dosing, Mrp4 levels were increased by 10- and 33-fold, respectively, in mice receiving empty liposomes. Immunohistochemistry revealed Mrp4 staining confined to centrilobular hepatocytes. Remarkably, Kupffer cell depletion completely prevented Mrp4 induction by APAP. Elevated plasma levels of TNF-alpha and IL-1beta were also prevented by Kupffer cell depletion. These findings show that Kupffer cells protect the liver from APAP toxicity and that Kupffer cell mediators released in response to APAP are likely responsible for the induction of Mrp4.
Figures

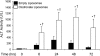


Similar articles
-
Effect of allyl alcohol on hepatic transporter expression: zonal patterns of expression and role of Kupffer cell function.Toxicol Appl Pharmacol. 2009 Apr 1;236(1):49-58. doi: 10.1016/j.taap.2009.01.007. Epub 2009 Jan 24. Toxicol Appl Pharmacol. 2009. PMID: 19371622 Free PMC article.
-
Protective role of Kupffer cells in acetaminophen-induced hepatic injury in mice.Chem Res Toxicol. 2002 Dec;15(12):1504-13. doi: 10.1021/tx0255976. Chem Res Toxicol. 2002. PMID: 12482232
-
Acquired resistance to acetaminophen hepatotoxicity is associated with induction of multidrug resistance-associated protein 4 (Mrp4) in proliferating hepatocytes.Toxicol Sci. 2008 Aug;104(2):261-73. doi: 10.1093/toxsci/kfn093. Epub 2008 May 8. Toxicol Sci. 2008. PMID: 18468992 Free PMC article.
-
Induction of Mrp3 and Mrp4 transporters during acetaminophen hepatotoxicity is dependent on Nrf2.Toxicol Appl Pharmacol. 2008 Jan 1;226(1):74-83. doi: 10.1016/j.taap.2007.08.022. Epub 2007 Aug 31. Toxicol Appl Pharmacol. 2008. PMID: 17935745 Free PMC article.
-
Lack of multidrug resistance-associated protein 4 (Mrp4) alters the kinetics of acetaminophen toxicity.Toxicol Rep. 2019 Aug 7;6:841-849. doi: 10.1016/j.toxrep.2019.08.005. eCollection 2019. Toxicol Rep. 2019. PMID: 31485416 Free PMC article.
Cited by
-
Pre-treatment twice with liposomal clodronate protects against acetaminophen hepatotoxicity through a pre-conditioning effect.Liver Res. 2020 Sep;4(3):145-152. doi: 10.1016/j.livres.2020.07.002. Epub 2020 Jul 29. Liver Res. 2020. PMID: 33042596 Free PMC article.
-
In vitro to in vivo extrapolation and species response comparisons for drug-induced liver injury (DILI) using DILIsym™: a mechanistic, mathematical model of DILI.J Pharmacokinet Pharmacodyn. 2012 Oct;39(5):527-41. doi: 10.1007/s10928-012-9266-0. Epub 2012 Aug 9. J Pharmacokinet Pharmacodyn. 2012. PMID: 22875368
-
Mechanistic Modelling of Drug-Induced Liver Injury: Investigating the Role of Innate Immune Responses.Gene Regul Syst Bio. 2017 May 30;11:1177625017696074. doi: 10.1177/1177625017696074. eCollection 2017. Gene Regul Syst Bio. 2017. PMID: 28615926 Free PMC article.
-
Monocyte-induced recovery of inflammation-associated hepatocellular dysfunction in a biochip-based human liver model.Sci Rep. 2016 Feb 23;6:21868. doi: 10.1038/srep21868. Sci Rep. 2016. PMID: 26902749 Free PMC article.
-
The Dual Role of Innate Immune Response in Acetaminophen-Induced Liver Injury.Biology (Basel). 2022 Jul 14;11(7):1057. doi: 10.3390/biology11071057. Biology (Basel). 2022. PMID: 36101435 Free PMC article. Review.
References
-
- Aleksunes LM, Scheffer GL, Jakowski AB, Pruimboom-Brees IM, Manautou JE. Coordinated expression of multidrug resistance-associated proteins (Mrps) in mouse liver during toxicant-induced injury. Toxicol Sci 89: 370–379, 2006. - PubMed
-
- Aleksunes LM, Slitt AM, Cherrington NJ, Thibodeau MS, Klaassen CD, Manautou JE. Differential expression of mouse hepatic transporter genes in response to acetaminophen and carbon tetrachloride. Toxicol Sci 83: 44–52, 2005. - PubMed
-
- Austyn JM, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol 11: 805–815, 1981. - PubMed
-
- Bai J, Lai L, Yeo HC, Goh BC, Tan TM. Multidrug resistance protein 4 (MRP4/ABCC4) mediates efflux of bimane-glutathione. Int J Biochem Cell Biol 36: 247–257, 2004. - PubMed
-
- Barnes SN, Aleksunes LM, Augustine L, Scheffer GL, Goedken M, Jakowski AB, Pruimboom-Brees IM, Cherrington NJ, Manautou JE. Induction of hepatobiliary efflux transporters in acetaminophen-induced acute liver failure cases. Drug Metab Dispos 35: 1963–1969, 2007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

