Detection of migration of locally implanted AC133+ stem cells by cellular magnetic resonance imaging with histological findings
- PMID: 18556461
- PMCID: PMC2518252
- DOI: 10.1096/fj.07-105676
Detection of migration of locally implanted AC133+ stem cells by cellular magnetic resonance imaging with histological findings
Abstract
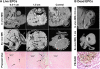
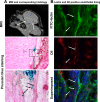
This study investigated the factors responsible for migration and homing of magnetically labeled AC133(+) cells at the sites of active angiogenesis in tumor. AC133(+) cells labeled with ferumoxide-protamine sulfate were mixed with either rat glioma or human melanoma cells and implanted in flank of nude mice. An MRI of the tumors including surrounding tissues was performed. Tumor sections were stained for Prussian blue (PB), platelet-derived growth factor (PDGF), hypoxia-inducible factor-1alpha (HIF-1alpha), stromal cell derived factor-1 (SDF-1), matrix metalloproteinase-2 (MMP-2), vascular endothelial growth factor (VEGF), and endothelial markers. Fresh snap-frozen strips from the central and peripheral parts of the tumor were collected for Western blotting. MRIs demonstrated hypointense regions at the periphery of the tumors where the PB(+)/AC133(+) cells were positive for endothelial cells markers. At the sites of PB(+)/AC133(+) cells, both HIF-1alpha and SDF-1 were strongly positive and PDGF and MMP-2 showed generalized expression in the tumor and surrounding tissues. There was no significant association of PB(+)/AC133(+) cell localization and VEGF expression in tumor cells. Western blot demonstrated strong expression of the SDF-1, MMP-2, and PDGF at the peripheral parts of the tumors. HIF-1alpha was expressed at both the periphery and central parts of the tumor. This work demonstrates that magnetically labeled cells can be used as probes for MRI and histological identification of administered cells.
Figures





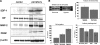
Similar articles
-
Magnetic resonance imaging and confocal microscopy studies of magnetically labeled endothelial progenitor cells trafficking to sites of tumor angiogenesis.Stem Cells. 2006 Mar;24(3):671-8. doi: 10.1634/stemcells.2005-0017. Epub 2005 Sep 22. Stem Cells. 2006. PMID: 16179427
-
MRI tracking of FePro labeled fresh and cryopreserved long term in vitro expanded human cord blood AC133+ endothelial progenitor cells in rat glioma.PLoS One. 2012;7(5):e37577. doi: 10.1371/journal.pone.0037577. Epub 2012 May 25. PLoS One. 2012. PMID: 22662174 Free PMC article.
-
The recruitment of exogenous endothelial progenitor cells in lung tumor model of nude mice.Chin J Cancer. 2010 Nov;29(11):952-8. doi: 10.5732/cjc.010.10194. Chin J Cancer. 2010. PMID: 20979695
-
AC133+ progenitor cells as gene delivery vehicle and cellular probe in subcutaneous tumor models: a preliminary study.BMC Biotechnol. 2009 Mar 27;9:28. doi: 10.1186/1472-6750-9-28. BMC Biotechnol. 2009. PMID: 19327159 Free PMC article.
-
Stromal cell-derived factor-1 stimulates vasculogenesis and enhances Ewing's sarcoma tumor growth in the absence of vascular endothelial growth factor.Int J Cancer. 2008 Aug 15;123(4):831-7. doi: 10.1002/ijc.23582. Int J Cancer. 2008. PMID: 18537159 Free PMC article.
Cited by
-
Nanotechnology applications for glioblastoma.Neurosurg Clin N Am. 2012 Jul;23(3):439-49. doi: 10.1016/j.nec.2012.04.006. Epub 2012 Jun 14. Neurosurg Clin N Am. 2012. PMID: 22748656 Free PMC article. Review.
-
Therapy with un-engineered naïve rat umbilical cord matrix stem cells markedly inhibits growth of murine lung adenocarcinoma.BMC Cancer. 2010 Oct 28;10:590. doi: 10.1186/1471-2407-10-590. BMC Cancer. 2010. PMID: 21029413 Free PMC article.
-
Magnetic nanoparticles: an emerging technology for malignant brain tumor imaging and therapy.Expert Rev Clin Pharmacol. 2012 Mar;5(2):173-86. doi: 10.1586/ecp.12.1. Expert Rev Clin Pharmacol. 2012. PMID: 22390560 Free PMC article. Review.
-
Human cord blood-derived AC133+ progenitor cells preserve endothelial progenitor characteristics after long term in vitro expansion.PLoS One. 2010 Feb 11;5(2):e9173. doi: 10.1371/journal.pone.0009173. PLoS One. 2010. PMID: 20161785 Free PMC article.
-
TIE-2 expressing monocytes in human cancers.Oncoimmunology. 2017 Mar 16;6(4):e1303585. doi: 10.1080/2162402X.2017.1303585. eCollection 2017. Oncoimmunology. 2017. PMID: 28507810 Free PMC article.
References
-
- Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. - PubMed
-
- Shi Q, Rafii S, Wu M H, Wijelath E S, Yu C, Ishida A, Fujita Y, Kothari S, Mohle R, Sauvage L R, Moore M A, Storb R F, Hammond W P. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362–367. - PubMed
-
- Samejima N, Yamazaki K. A study on the vascular proliferation in tissues around the tumor in breast cancer. Jpn J Surg. 1988;18:235–242. - PubMed
-
- Ellis L M, Liu W, Ahmad S A, Fan F, Jung Y D, Shaheen R M, Reinmuth N. Overview of angiogenesis: Biologic implications for antiangiogenic therapy. Semin Oncol. 2001;28:94–104. - PubMed
-
- Liekens S, De Clercq E, Neyts J. Angiogenesis: regulators and clinical applications. Biochem Pharmacol. 2001;61:253–270. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

