GRP78 upregulation by atheroprone shear stress via p38-, alpha2beta1-dependent mechanism in endothelial cells
- PMID: 18556570
- PMCID: PMC2723835
- DOI: 10.1161/ATVBAHA.108.167999
GRP78 upregulation by atheroprone shear stress via p38-, alpha2beta1-dependent mechanism in endothelial cells
Abstract
Objective: The initiation of atherosclerosis is in part dependent on the hemodynamic shear stress environment promoting a proinflammatory phenotype of the endothelium. Previous studies demonstrated increased expression of ER stress protein and unfolded protein response (UPR) regulator, GRP78, within all vascular cells in atherosclerotic lesions and its regulation in the endothelium by several atherosclerotic stressors; however, regulation of GRP78 by shear stress directly has not been established.
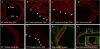
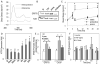
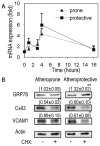
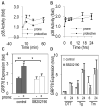
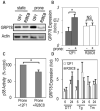
Method and results: Using an in vitro model to simulate human arterial shear stress waveforms, atheroprone or atheroprotective flow was applied to human endothelial cells. GRP78 was found to be significantly upregulated (3-fold) in a sustained manner under atheroprone, but not atheroprotective flow up to 24 hours. This response was dependent on both sustained activation of p38, as well integrin alpha2beta1. Increased GRP78 correlated with the activation of the ER stress sensing element (ERSE1) promoter by atheroprone flow as a marker of the UPR. Shear stress regulated GRP78 through increased protein stability when compared to other flow regulated proteins, such as connexin-43 and vascular cell adhesion molecule (VCAM)-1. Increased endothelial expression of GRP78 was also observed in atheroprone versus atheroprotective regions of C57BL6 mice.
Conclusions: This study supports a role of the hemodynamic environment in preferentially inducing GRP78 and the UPR in atheroprone regions, before lesion development, and suggests a potential atheroprotective (ie, prosurvival), compensatory effect in response to ER stress within atherosclerotic lesions.
Figures
References
-
- Passerini AG, Polacek DC, Shi C, Francesco NM, Manduchi E, Grant GR, Pritchard WF, Powell S, Chang GY, Stoeckert CJ, Jr, Davies PF. Coexisting proinflammatory and antioxidative endothelial transcription profiles in a disturbed flow region of the adult porcine aorta. Proc Natl Acad Sci U S A. 2004;101:2482–2487. - PMC - PubMed
-
- Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vaughn S, Blackman BR, Kamm RD, Garcia-Cardena G, Gimbrone MA., Jr Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc Natl Acad Sci U S A. 2004;101:14871–14876. - PMC - PubMed
-
- Zhou J, Werstuck GH, Lhotak S, de Koning AB, Sood SK, Hossain GS, Moller J, Ritskes-Hoitinga M, Falk E, Dayal S, Lentz SR, Austin RC. Association of multiple cellular stress pathways with accelerated atherosclerosis in hyperhomocysteinemic apolipoprotein E-deficient mice. Circulation. 2004;110:207–213. - PubMed
-
- Bhattacharjee G, Ahamed J, Pedersen B, El-Sheikh A, Mackman N, Ruf W, Liu C, Edgington TS. Regulation of tissue factor--mediated initiation of the coagulation cascade by cell surface grp78. Arterioscler Thromb Vasc Biol. 2005;25:1737–1743. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

