Thy-1 promoter hypermethylation: a novel epigenetic pathogenic mechanism in pulmonary fibrosis
- PMID: 18556592
- PMCID: PMC2574530
- DOI: 10.1165/rcmb.2007-0322OC
Thy-1 promoter hypermethylation: a novel epigenetic pathogenic mechanism in pulmonary fibrosis
Abstract
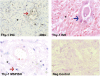
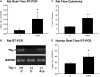
Mechanisms regulating myofibroblastic differentiation of fibroblasts within fibroblastic foci in idiopathic pulmonary fibrosis (IPF) remain unclear. Epigenetic processes, including DNA methylation, produce heritable but potentially reversible changes in DNA or its associated proteins and are prominent in development and oncogenesis. We have shown that Thy-1 suppresses myofibroblastic differentiation of lung fibroblasts and that fibroblasts in fibroblastic foci are Thy-1(-). Epigenetic down-regulation of Thy-1 has been demonstrated in cellular transformation and clinical cancer. We hypothesized that epigenetic regulation of Thy-1 affects the lung fibroblast fibrogenic phenotype. RT-PCR, methylation-specific PCR (MSP), and bisulfite genomic sequencing were used to determine the methylation status of the Thy-1 promoter in Thy-1(+) and Thy-1(-) lung fibroblasts, and MSP-in situ hybridization (MSPISH) was performed on fibrotic tissue. Thy-1 gene expression is absent in Thy-1(-) human and rat fibroblasts despite intact Thy-1 genomic DNA. Cytosine-guanine islands in the Thy-1 gene promoter are hypermethylated in Thy-1(-), but not Thy-1(+), fibroblasts. RT-PCR and MSP demonstrate that, in IPF samples in which Thy-1 expression is absent, the Thy-1 promoter region is methylated, whereas in lung samples retaining Thy-1 expression, the promoter region is unmethylated. MSPISH confirms methylation of the Thy-1 promoter in fibroblastic foci in IPF. Treatment with DNA methyltransferase inhibitors restores Thy-1 expression in Thy-1(-) fibroblasts. Epigenetic regulation of Thy-1 is a novel and potentially reversible mechanism in fibrosis that may offer the possibility of new therapeutic options.
Figures





References
-
- Pardo A, Selman M. Idiopathic pulmonary fibrosis: new insights in its pathogenesis. Int J Biochem Cell Biol 2002;34:1534–1538. - PubMed
-
- Kuhn C, Boldt J, King TE, Crouch E, Vartio T, McDonald JA. An immunohistochemical study of architectural remodeling and connective tissue synthesis in pulmonary fibrosis. Am Rev Respir Dis 1989;140:1693–1703. - PubMed
-
- Phan SH. Fibroblast phenotypes in pulmonary fibrosis. Am J Respir Cell Mol Biol 2003;29(3, Suppl)S87–S92. - PubMed
-
- Rege TA, Hagood JS. Thy-1 as a regulator of cell-cell and cell-matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. FASEB J 2006;20:1045–1054. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

