Triiodo-L-thyronine rapidly stimulates alveolar fluid clearance in normal and hyperoxia-injured lungs
- PMID: 18556623
- PMCID: PMC2542429
- DOI: 10.1164/rccm.200709-1429OC
Triiodo-L-thyronine rapidly stimulates alveolar fluid clearance in normal and hyperoxia-injured lungs
Abstract
Rationale: Edema fluid resorption is critical for gas exchange and requires active epithelial ion transport by Na, K-ATPase and other ion transport proteins.
Objectives: In this study, we sought to determine if alveolar fluid clearance (AFC) is stimulated by 3,3',5 triiodo-L-thyronine (T(3)).
Methods: AFC was measured in in situ ventilated lungs and ex vivo isolated lungs by instilling isosmolar 5% bovine serum albumin solution with fluorescein-labeled albumin tracer and measuring the change in fluorescein isothiocyanate-albumin concentration over time.
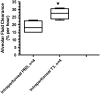
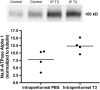
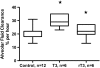
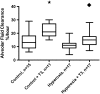
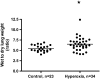
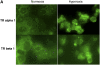
Measurements and main results: Systemic treatment with intraperitoneal injections of T(3) for 3 consecutive days increased AFC by 52.7% compared with phosphate-buffered saline-injected control rats. Membranes prepared from alveolar epithelial cells from T(3)-treated rats had higher Na, K-ATPase hydrolytic activity. T(3) (10(-6) M), but not reverse T(3) (3,3',5' triiodo-L-thyronine), applied to the alveolar space increased AFC by 31.8% within 1.5 hours. A 61.5% increase in AFC also occurred by airspace instillation of T(3) in ex vivo isolated lungs, suggesting a direct effect of T(3) on the alveolar epithelium. Exposure of rats to an oxygen concentration of greater than 95% for 60 hours increased wet-to-dry lung weights and decreased AFC, whereas the expression of thyroid receptor was not markedly changed. Airspace T(3) rapidly restored the AFC in rat lungs with hyperoxia-induced lung injury.
Conclusions: Airspace T(3) rapidly stimulates AFC by direct effects on the alveolar epithelium in rat lungs with and without lung injury.
Figures


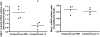


Similar articles
-
Lipoxin A(4) activates alveolar epithelial sodium channel, Na,K-ATPase, and increases alveolar fluid clearance.Am J Respir Cell Mol Biol. 2013 May;48(5):610-8. doi: 10.1165/rcmb.2012-0274OC. Am J Respir Cell Mol Biol. 2013. PMID: 23470626
-
Thyroid hormone stimulates Na-K-ATPase activity and its plasma membrane insertion in rat alveolar epithelial cells.Am J Physiol Lung Cell Mol Physiol. 2003 Sep;285(3):L762-72. doi: 10.1152/ajplung.00376.2002. Epub 2003 May 9. Am J Physiol Lung Cell Mol Physiol. 2003. PMID: 12740220
-
Dopamine restores lung ability to clear edema in rats exposed to hyperoxia.Am J Respir Crit Care Med. 1999 Feb;159(2):626-33. doi: 10.1164/ajrccm.159.2.9805016. Am J Respir Crit Care Med. 1999. PMID: 9927383
-
Role and regulation of lung Na,K-ATPase.Cell Mol Biol (Noisy-le-grand). 2001 Mar;47(2):347-61. Cell Mol Biol (Noisy-le-grand). 2001. PMID: 11355011 Review.
-
Thyroid hormone rapidly stimulates alveolar Na,K-ATPase by activation of phosphatidylinositol 3-kinase.Curr Opin Endocrinol Diabetes Obes. 2007 Oct;14(5):416-20. doi: 10.1097/MED.0b013e3282f02ae8. Curr Opin Endocrinol Diabetes Obes. 2007. PMID: 17940473 Review.
Cited by
-
Protein expression profile of rat type two alveolar epithelial cells during hyperoxic stress and recovery.Am J Physiol Lung Cell Mol Physiol. 2013 Nov 1;305(9):L604-14. doi: 10.1152/ajplung.00079.2013. Epub 2013 Sep 6. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 24014686 Free PMC article.
-
Physiological Role and Use of Thyroid Hormone Metabolites - Potential Utility in COVID-19 Patients.Front Endocrinol (Lausanne). 2021 Apr 26;12:587518. doi: 10.3389/fendo.2021.587518. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33981284 Free PMC article. Review.
-
Endoplasmic reticulum stress decreases intracellular thyroid hormone activation via an eIF2a-mediated decrease in type 2 deiodinase synthesis.Mol Endocrinol. 2011 Dec;25(12):2065-75. doi: 10.1210/me.2011-1061. Epub 2011 Nov 3. Mol Endocrinol. 2011. PMID: 22053000 Free PMC article.
-
Maresin Conjugates in Tissue Regeneration 1 improves alveolar fluid clearance by up-regulating alveolar ENaC, Na, K-ATPase in lipopolysaccharide-induced acute lung injury.J Cell Mol Med. 2020 Apr;24(8):4736-4747. doi: 10.1111/jcmm.15146. Epub 2020 Mar 11. J Cell Mol Med. 2020. PMID: 32160403 Free PMC article.
-
Effects of Thyroid Hormone on Tissue Hypoxia: Relevance to Sepsis Therapy.J Clin Med. 2021 Dec 14;10(24):5855. doi: 10.3390/jcm10245855. J Clin Med. 2021. PMID: 34945151 Free PMC article. Review.
References
-
- Matthay MA, Clerici C, Saumon G. Invited review: active fluid clearance from the distal air spaces of the lung. J Appl Physiol 2002;93:1533–1541. - PubMed
-
- Kemp PJ, Kim KJ. Spectrum of ion channels in alveolar epithelial cells: implications for alveolar fluid balance. Am J Physiol Lung Cell Mol Physiol 2004;287:L460–L464. - PubMed
-
- Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev 2002;82:569–600. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

