Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1
- PMID: 18556627
- PMCID: PMC2542433
- DOI: 10.1164/rccm.200803-380OC
Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1
Erratum in
- Am J Respir Crit Care Med. 2009 Apr 1;179(7):624
Retraction in
-
Retraction: Decline in NRF2-regulated Antioxidants in Chronic Obstructive Pulmonary Disease Lungs Due to Loss of Its Positive Regulator, DJ-1; Heightened Endoplasmic Reticulum Stress in the Lungs of Patients with Chronic Obstructive Pulmonary Disease: The Role of Nrf2-Regulated Proteasomal Activity.Am J Respir Crit Care Med. 2016 Feb 1;193(3):344. doi: 10.1164/rccm.1933retraction. Am J Respir Crit Care Med. 2016. PMID: 26829430 Free PMC article. No abstract available.
Expression of concern in
-
Expression of concern: decline in NRF2-regulated antioxidants in COPD lungs due to loss of its positive regulator, and heightened endoplasmic reticulum stress in the lungs of patients with COPD.Am J Respir Crit Care Med. 2014 Nov 15;190(10):1200. doi: 10.1164/rccm.190101200. Am J Respir Crit Care Med. 2014. PMID: 25398118 Free PMC article. No abstract available.
Abstract
Rationale: Oxidative stress is a key contributor in chronic obstructive pulmonary disease (COPD) pathogenesis caused by cigarette smoking. NRF2, a redox-sensitive transcription factor, dissociates from its inhibitor, KEAP1, to induce antioxidant expression that inhibits oxidative stress.
Objectives: To determine the link between severity of COPD, oxidative stress, and NRF2-dependent antioxidant levels in the peripheral lung tissue of patients with COPD.
Methods: We assessed the expression of NRF2, NRF2-dependent antioxidants, regulators of NRF2 activity, and oxidative damage in non-COPD (smokers and former smokers) and smoker COPD lungs (mild and advanced). Cigarette smoke-exposed human lung epithelial cells (Beas2B) and mice were used to understand the mechanisms.
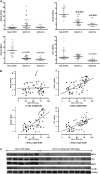
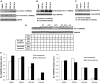
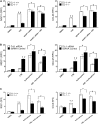
Measurements and main results: When compared with non-COPD lungs, the COPD patient lungs showed (1) marked decline in NRF2-dependent antioxidants and glutathione levels, (2) increased oxidative stress markers, (3) significant decrease in NRF2 protein with no change in NRF2 mRNA levels, and (4) similar KEAP1 but significantly decreased DJ-1 levels (a protein that stabilizes NRF2 protein by impairing KEAP1-dependent proteasomal degradation of NRF2). Exposure of Bea2B cells to cigarette smoke caused oxidative modification and enhanced proteasomal degradation of DJ-1 protein. Disruption of DJ-1 in mouse lungs, mouse embryonic fibroblasts, and Beas2B cells lowered NRF2 protein stability and impaired antioxidant induction in response to cigarette smoke. Interestingly, targeting KEAP1 by siRNA or the small-molecule activator sulforaphane restored induction of NRF2-dependent antioxidants in DJ-1-disrupted cells in response to cigarette smoke.
Conclusions: NRF2-dependent antioxidants and DJ-1 expression was negatively associated with severity of COPD. Therapy directed toward enhancing NRF2-regulated antioxidants may be a novel strategy for attenuating the effects of oxidative stress in the pathogenesis of COPD.
Figures

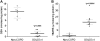

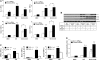
Comment in
-
Defective antioxidant gene regulation in COPD: a case for broccoli.Am J Respir Crit Care Med. 2008 Sep 15;178(6):552-4. doi: 10.1164/rccm.200806-956ED. Am J Respir Crit Care Med. 2008. PMID: 18755929 No abstract available.
Similar articles
-
Heightened endoplasmic reticulum stress in the lungs of patients with chronic obstructive pulmonary disease: the role of Nrf2-regulated proteasomal activity.Am J Respir Crit Care Med. 2009 Dec 15;180(12):1196-207. doi: 10.1164/rccm.200903-0324OC. Epub 2009 Oct 1. Am J Respir Crit Care Med. 2009. Retraction in: Am J Respir Crit Care Med. 2016 Feb 1;193(3):344. doi: 10.1164/rccm.1933retraction. PMID: 19797762 Free PMC article. Retracted.
-
Decreased DJ-1 leads to impaired Nrf2-regulated antioxidant defense and increased UV-A-induced apoptosis in corneal endothelial cells.Invest Ophthalmol Vis Sci. 2014 Jul 31;55(9):5551-60. doi: 10.1167/iovs.14-14580. Invest Ophthalmol Vis Sci. 2014. PMID: 25082883 Free PMC article.
-
Characterization of the Potent, Selective Nrf2 Activator, 3-(Pyridin-3-Ylsulfonyl)-5-(Trifluoromethyl)-2H-Chromen-2-One, in Cellular and In Vivo Models of Pulmonary Oxidative Stress.J Pharmacol Exp Ther. 2017 Oct;363(1):114-125. doi: 10.1124/jpet.117.241794. Epub 2017 Aug 8. J Pharmacol Exp Ther. 2017. PMID: 28790194
-
NRF2 targeting: a promising therapeutic strategy in chronic obstructive pulmonary disease.Trends Mol Med. 2011 Jul;17(7):363-71. doi: 10.1016/j.molmed.2011.02.006. Epub 2011 Apr 1. Trends Mol Med. 2011. PMID: 21459041 Review.
-
Markers of anti-oxidant response in tobacco smoke exposed subjects: a data-mining review.Pulm Pharmacol Ther. 2010 Dec;23(6):482-92. doi: 10.1016/j.pupt.2010.05.006. Epub 2010 Jun 1. Pulm Pharmacol Ther. 2010. PMID: 20594977 Review.
Cited by
-
Activating the Nrf2-mediated antioxidant response element restores barrier function in the alveolar epithelium of HIV-1 transgenic rats.Am J Physiol Lung Cell Mol Physiol. 2013 Aug 1;305(3):L267-77. doi: 10.1152/ajplung.00288.2012. Epub 2013 Jun 7. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 23748533 Free PMC article.
-
Inflammation in chronic obstructive pulmonary disease and its role in cardiovascular disease and lung cancer.Clin Transl Med. 2015 Dec;4(1):68. doi: 10.1186/s40169-015-0068-z. Epub 2015 Jul 29. Clin Transl Med. 2015. PMID: 26220864 Free PMC article.
-
NRF2 and the Phase II Response in Acute Stress Resistance Induced by Dietary Restriction.J Clin Exp Pathol. 2012 Jun 19;S4(4):7329. doi: 10.4172/2161-0681.S4-004. J Clin Exp Pathol. 2012. PMID: 23505614 Free PMC article.
-
NFE2L2 pathway polymorphisms and lung function decline in chronic obstructive pulmonary disease.Physiol Genomics. 2012 Aug 1;44(15):754-63. doi: 10.1152/physiolgenomics.00027.2012. Epub 2012 Jun 12. Physiol Genomics. 2012. PMID: 22693272 Free PMC article.
-
Geroprotectors as a novel therapeutic strategy for COPD, an accelerating aging disease.Int J Chron Obstruct Pulmon Dis. 2012;7:641-52. doi: 10.2147/COPD.S28250. Epub 2012 Sep 21. Int J Chron Obstruct Pulmon Dis. 2012. PMID: 23055713 Free PMC article. Review.
References
-
- Barnes PJ. Mediators of chronic obstructive pulmonary disease. Pharmacol Rev 2004;56:515–548. - PubMed
-
- Yoshida T, Tuder RM. Pathobiology of cigarette smoke-induced chronic obstructive pulmonary disease. Physiol Rev 2007;87:1047–1082. - PubMed
-
- Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL, Held LS, Schmid V, Buist S. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J 2006;27:397–412. - PubMed
-
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007;370:765–773. - PubMed
-
- Shapiro SD, Ingenito EP. The pathogenesis of chronic obstructive pulmonary disease: advances in the past 100 years. Am J Respir Cell Mol Biol 2005;32:367–372. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

